Deep Scientific Insights on Panitumumab's R&D Progress, Mechanism of Action, and Drug Target
Panitumumab's R&D Progress
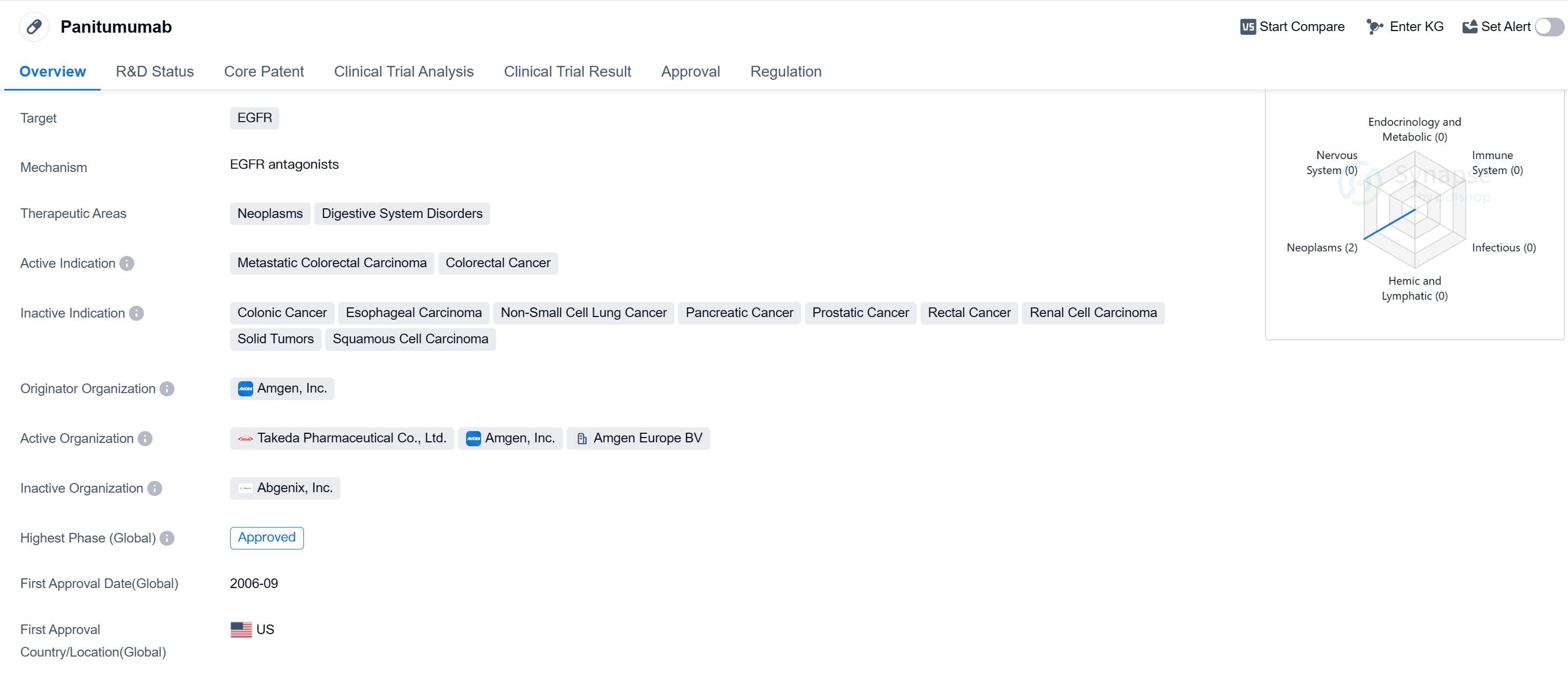
Panitumumab is a monoclonal antibody drug that targets the epidermal growth factor receptor (EGFR). It is primarily used in the treatment of neoplasms and digestive system disorders. The active indications for this drug include metastatic colorectal carcinoma and colorectal cancer.
The drug was developed by Amgen, Inc., a renowned pharmaceutical company. It received its highest phase of approval globally. The first approval for panitumumab was granted in September 2006 in the United States, making it available for patients in that country.
However, it is important to note that the highest phase of approval for panitumumab in China is still pending. This suggests that the drug is currently undergoing regulatory processes in China and has not yet been approved for use in the country.
Panitumumab received accelerated approval, which means that it was granted regulatory approval based on promising early clinical trial results. This type of approval is typically given to drugs that address serious or life-threatening conditions and fulfill an unmet medical need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Panitumumab: EGFR antagonists
EGFR antagonists are a type of medication that specifically target and inhibit the activity of the epidermal growth factor receptor (EGFR). The EGFR is a protein found on the surface of cells and plays a crucial role in regulating cell growth, division, and survival. However, in certain diseases, such as certain types of cancer, the EGFR can become overactive and promote uncontrolled cell growth.
EGFR antagonists work by binding to the EGFR and blocking its activation. By doing so, they prevent the EGFR from sending signals that promote cell growth and division. This inhibition of EGFR activity can help slow down or stop the growth of cancer cells.
EGFR antagonists are commonly used in the treatment of various types of cancer, including lung cancer, colorectal cancer, and head and neck cancer. They can be administered through different routes, such as oral tablets or intravenous infusion, depending on the specific medication.
It is important to note that EGFR antagonists may have side effects, which can vary depending on the specific medication and individual patient factors. Common side effects may include skin rash, diarrhea, nausea, and fatigue. Close monitoring and management of these side effects are typically done by healthcare professionals during treatment.
Overall, EGFR antagonists are valuable therapeutic agents in biomedicine, particularly in the field of oncology, as they target a specific molecular pathway involved in cancer cell growth and proliferation.
Drug Target R&D Trends for Panitumumab
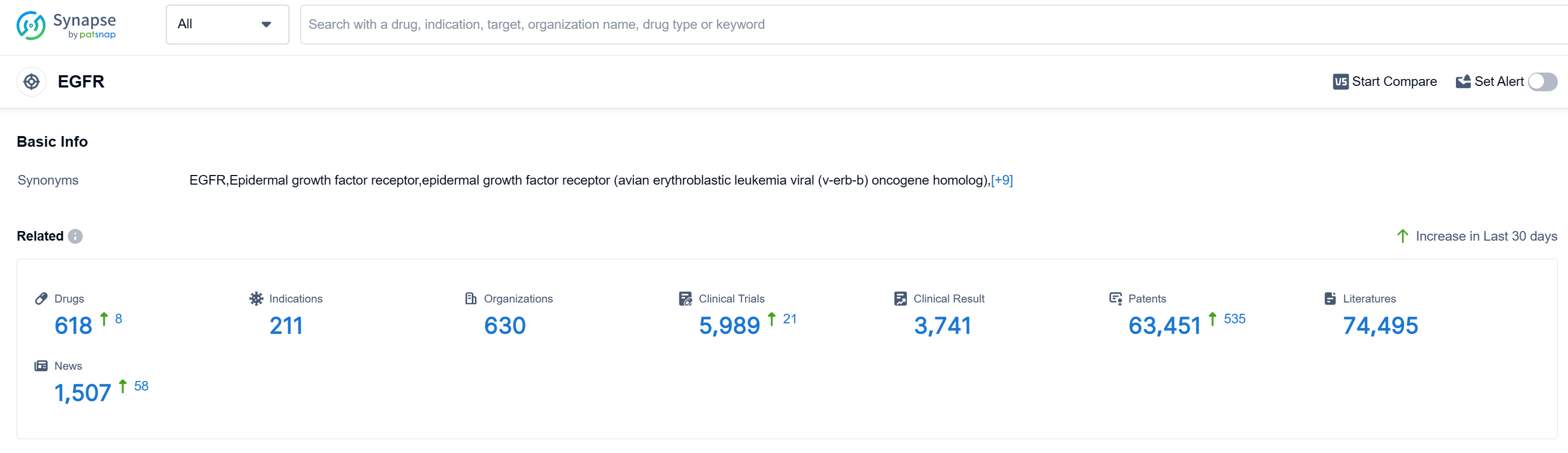
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 620 EGFR drugs worldwide, from 630 organizations, covering 210 indications, and conducting 5995 clinical trials.
The analysis of the current competitive landscape of target EGFR reveals that AstraZeneca PLC is the leading company with the highest stage of development. The most common approved indication is Non-Small Cell Lung Cancer. Small molecule drugs, growth factors, and monoclonal antibodies are progressing rapidly. China is the fastest-developing country under this target, followed by the United States. The future development of target EGFR is expected to see continued growth and competition, with companies and countries focusing on developing innovative drugs and expanding indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, panitumumab is a monoclonal antibody drug developed by Amgen, Inc. It targets the EGFR and is primarily used in the treatment of neoplasms and digestive system disorders. It has received approval for the treatment of metastatic colorectal carcinoma and colorectal cancer in the United States. However, its highest phase of approval in China is still pending. The drug received accelerated approval, indicating its potential to address serious medical conditions.