Nizatidine Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs, Mechanisms of Action, and Drug Target
Nizatidine's R&D Progress
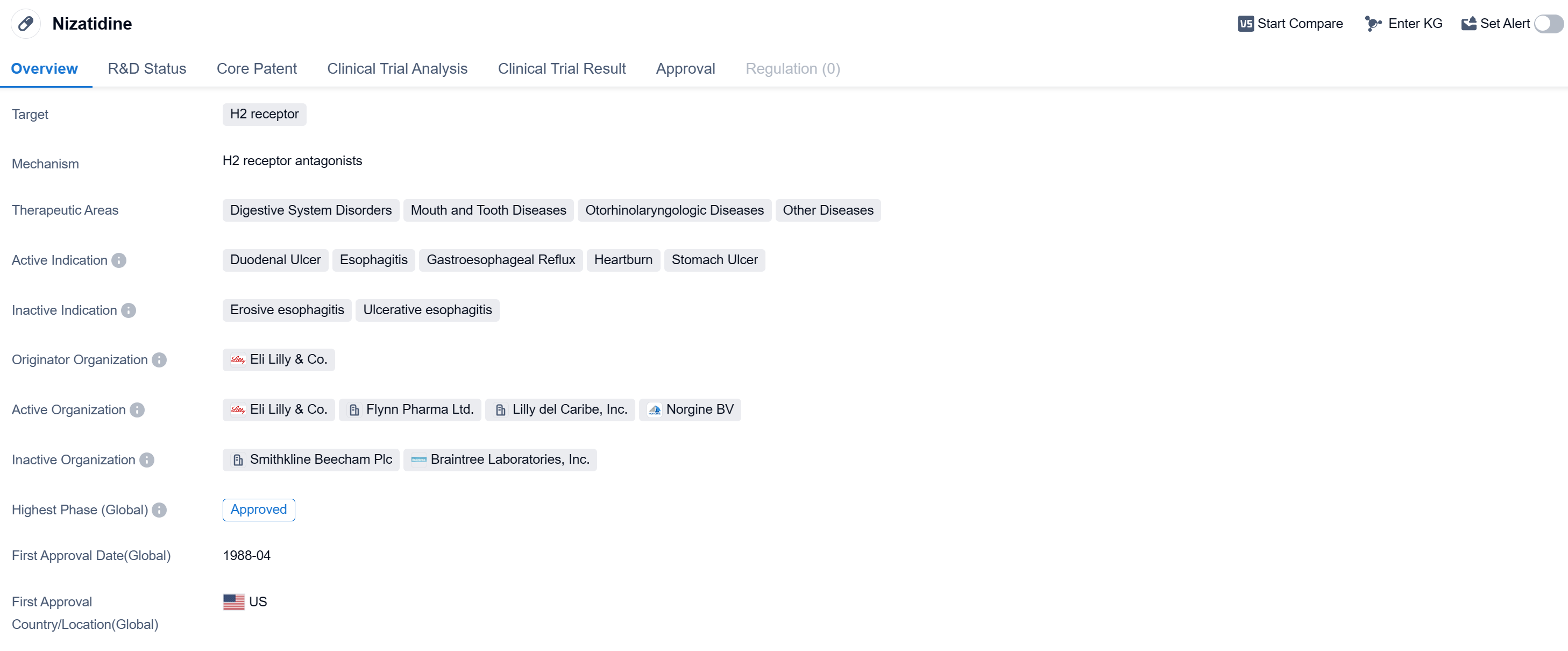
Nizatidine is a small molecule drug that belongs to the class of H2 receptor antagonists. It is primarily used in the treatment of various digestive system disorders, mouth and tooth diseases, otorhinolaryngologic diseases, and other related conditions. The drug has been approved for the treatment of duodenal ulcer, esophagitis, gastroesophageal reflux, heartburn, and stomach ulcer.
Nizatidine was developed by Eli Lilly & Co., a renowned pharmaceutical organization. It received its first approval in the United States in April 1988, making it available for use in patients. The drug has also obtained approvals in other countries, indicating its global acceptance and recognition.
As a small molecule drug, Nizatidine works by selectively blocking the H2 receptors, which are primarily found in the stomach lining. By inhibiting these receptors, the drug reduces the production of stomach acid, thereby alleviating symptoms associated with digestive disorders such as ulcers, reflux, and heartburn. This mechanism of action makes Nizatidine an effective treatment option for patients suffering from these conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Nizatidine: H2 receptor antagonists
H2 receptor antagonists are a type of medication that block the action of histamine on the H2 receptors in the stomach. From a biomedical perspective, these drugs are commonly used to reduce the production of stomach acid and are primarily used in the treatment of conditions such as gastroesophageal reflux disease (GERD), peptic ulcers, and gastritis. By blocking the H2 receptors, these antagonists help to decrease the secretion of gastric acid, providing relief from symptoms such as heartburn, acid reflux, and stomach pain. Some examples of H2 receptor antagonists include ranitidine, famotidine, and cimetidine.
Drug Target R&D Trends for Nizatidine
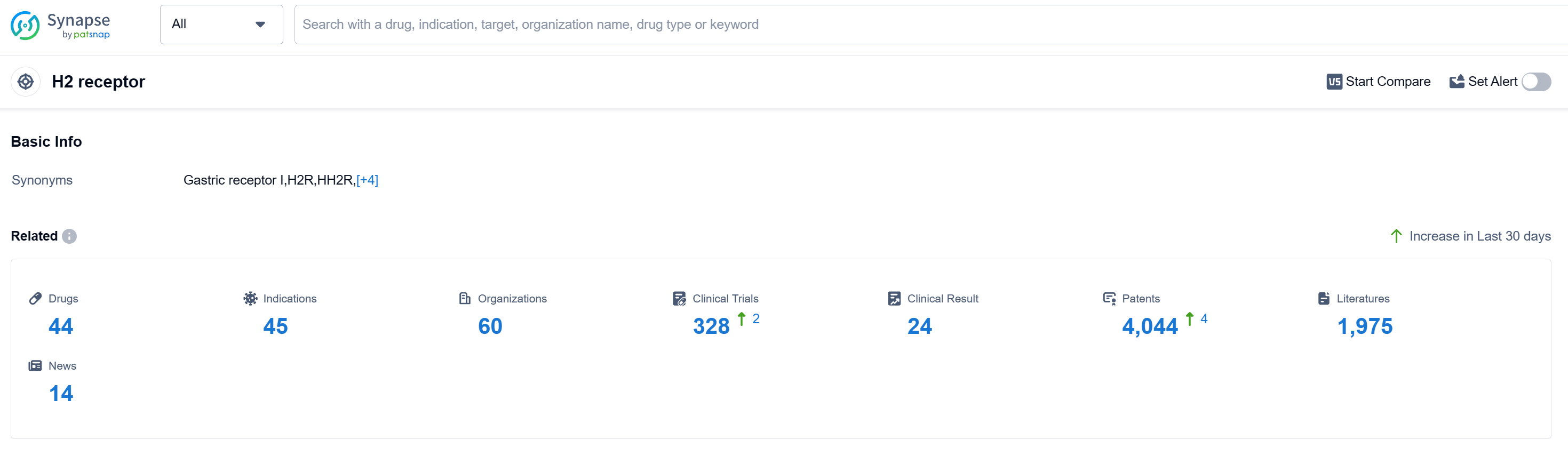
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 44 H2 receptor drugs worldwide, from 60 organizations, covering 45 indications, and conducting 328 clinical trials.
The analysis of the target H2 receptor in the pharmaceutical industry reveals that multiple companies are growing rapidly and have successfully developed drugs that have been approved. The most common approved indication is Stomach Ulcer, and the drug types progressing most rapidly are Small molecule drugs and Recombinant proteins. China is showing significant progress in the development of drugs targeting the H2 receptor. The presence of biosimilars indicates intense competition around the innovative drugs. Further analysis of R&D progress, research and development institutions, and progress in China requires additional data to provide a more comprehensive understanding of the current competitive landscape and future development of the target H2 receptor.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Nizatidine is a small molecule drug developed by Eli Lilly & Co. It acts as an H2 receptor antagonist and is approved for the treatment of various digestive system disorders, mouth and tooth diseases, otorhinolaryngologic diseases, and other related conditions. With its first approval in the United States in 1988, Nizatidine has established itself as a reliable and effective treatment option for patients suffering from duodenal ulcer, esophagitis, gastroesophageal reflux, heartburn, and stomach ulcer.