Analysis on the Research Progress of HSP90 inhibitors
Heat shock protein 90 (HSP90) is an ATP-dependent molecular chaperone essential in eukaryotic organisms. It is ubiquitously present in both prokaryotic and eukaryotic cells, excluding archaea, and is one of the core members of the heat shock protein family. In normal cells, HSP90 makes up 1-2% of the total proteins, this percentage increasing to 4-6% under stressful conditions. Moreover, HSP90 is highly conserved evolutionarily, with its function being consistent across cells of different species origins.
Molecular chaperone proteins in cells play a critical role in protein folding through their binding with substrate proteins. HSP90 is such a molecular chaperone protein, capable of high activation in the harsh tumour environment of various solid tumours, yet remains relatively dormant in normal tissues. Therefore, it is considered a potential target for tumour drug development. In addition, HSP90 serves as a downstream modulator for the signaling of Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-17A (IL-17A).
HSP90 Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 18 Sep 2023, there are a total of 55 HSP90 drugs worldwide, from 67 organizations, covering 78 indications, and conducting 224 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.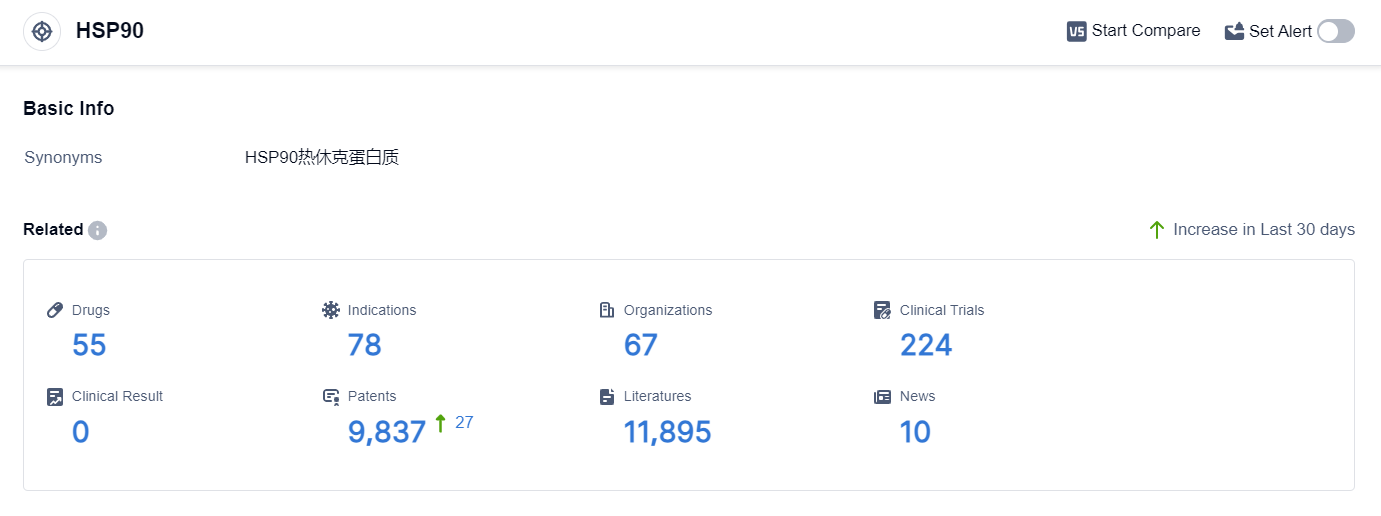
The analysis of the target HSP90 reveals a competitive landscape with companies like Wakamoto Pharmaceutical Co., Ltd., D. Western Therapeutics Institute, Inc., and Samus Therapeutics LLC leading the way in R&D efforts.
The indications with approved drugs include Acute Myeloid Leukemia, Myelodysplastic Syndromes, and Glaucoma. Small molecule drugs, including small molecule-drug conjugates, are progressing rapidly, indicating the primary focus of R&D efforts.
The European Union, the United Kingdom, and Japan are the fastest-developing regions, with emerging markets in China and South Korea. Overall, the target HSP90 shows promising potential for the development of innovative drugs in various indications, with a strong focus on small molecule drugs.
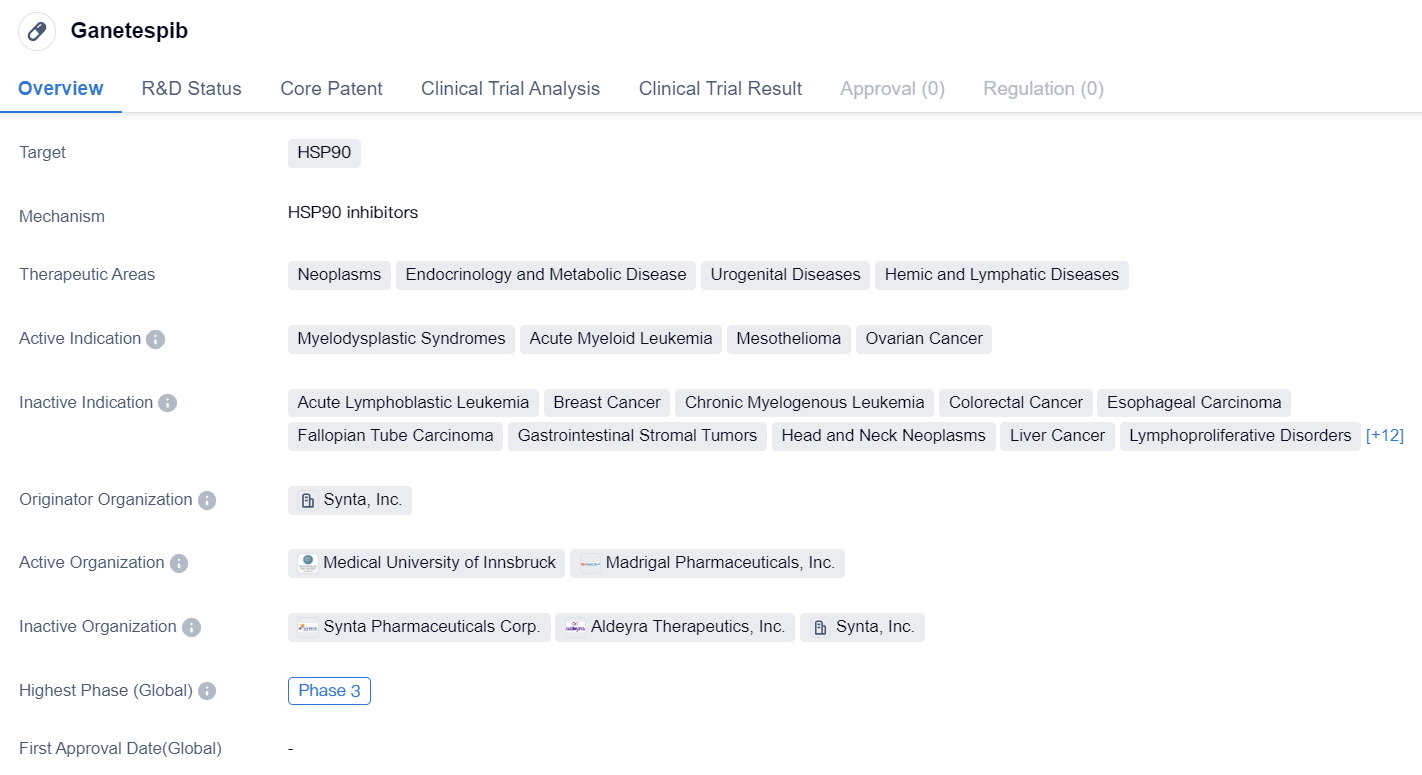
HSP90 inhibitors entering Phase III clinical trials: Ganetespib
Ganetespib is a small molecule drug that falls under the category of biomedicine. It specifically targets HSP90, a protein that plays a crucial role in the folding and stabilization of other proteins. By inhibiting HSP90, Ganetespib aims to disrupt the function of various proteins involved in disease processes.
The therapeutic areas in which Ganetespib is being explored include neoplasms (abnormal growth of cells), endocrinology and metabolic diseases, urogenital diseases, and hemic and lymphatic diseases. These broad therapeutic areas suggest that Ganetespib has the potential to be effective in treating a wide range of conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Currently, Ganetespib is being investigated for its efficacy in treating several specific indications. These include myelodysplastic syndromes (a group of disorders characterized by abnormal blood cell production), acute myeloid leukemia (a type of cancer that affects the blood and bone marrow), mesothelioma (a rare cancer that affects the lining of the lungs, abdomen, or heart), and ovarian cancer.
The drug is developed by Synta, Inc., an originator organization in the pharmaceutical industry. Synta, Inc. is responsible for the discovery and development of Ganetespib, and they hold the rights to the drug.
In terms of its development stage, Ganetespib has reached Phase 3 of clinical trials. This indicates that the drug has already undergone extensive testing in preclinical and early clinical stages and has shown promising results. Phase 3 trials involve a larger number of participants and aim to further evaluate the drug's safety and effectiveness compared to existing treatments or a placebo.
In summary, Ganetespib is a small molecule drug that targets HSP90 and is being investigated for its potential in treating various neoplasms, endocrinology and metabolic diseases, urogenital diseases, and hemic and lymphatic diseases. It is currently in Phase 3 clinical trials and is being developed by Synta, Inc.
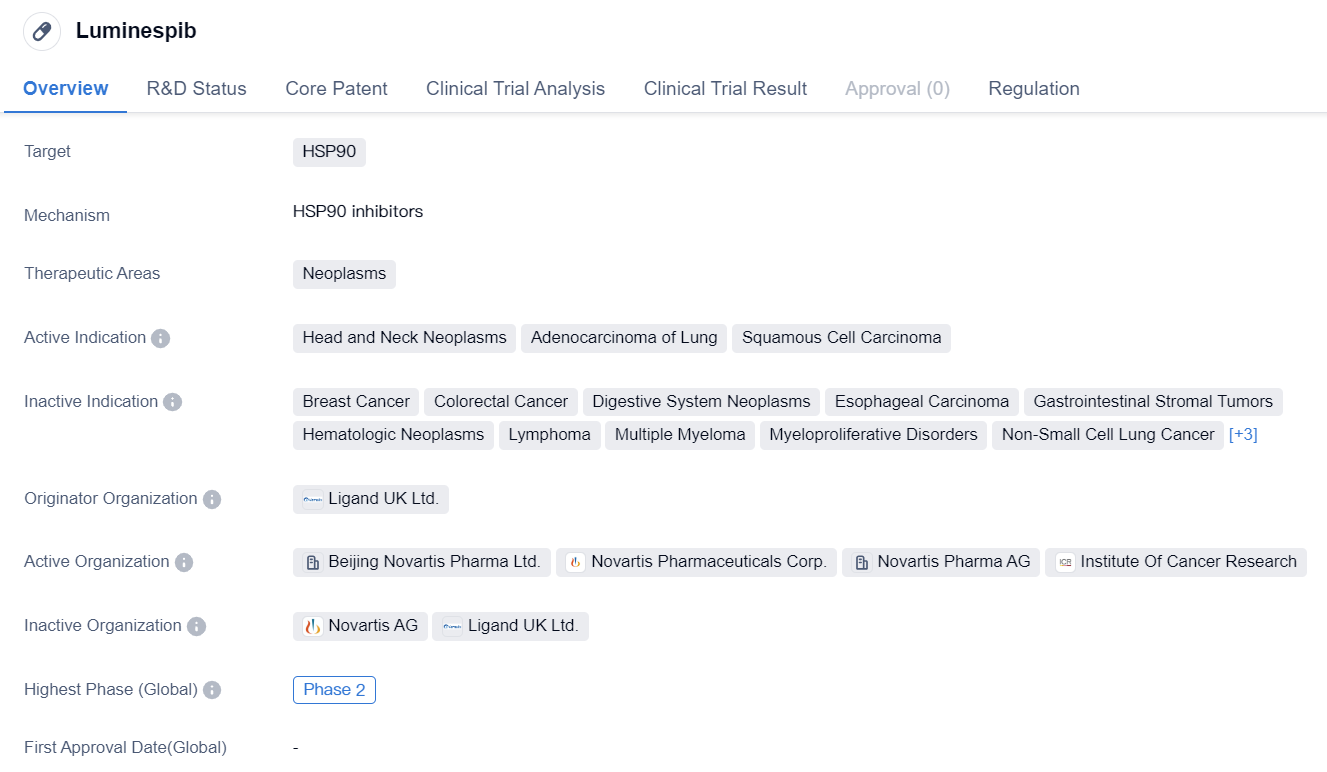
Luminespib
Luminespib is a small molecule drug that falls under the category of biomedicine. It specifically targets HSP90, a protein involved in various cellular processes. The drug is primarily developed for the treatment of neoplasms, which are abnormal growths of tissue commonly referred to as tumors.
Luminespib has shown potential in treating specific types of neoplasms, including head and neck neoplasms, adenocarcinoma of the lung, and squamous cell carcinoma. These indications suggest that the drug may be effective in combating various forms of cancer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The drug's originator organization is Ligand UK Ltd., a pharmaceutical company involved in the research and development of innovative therapies. This suggests that Luminespib is a product of extensive scientific research and development efforts.
In terms of its development stage, Luminespib has reached Phase 2, which is considered a significant milestone in the drug development process. Phase 2 trials involve testing the drug on a larger group of patients to evaluate its safety and efficacy. The fact that Luminespib has reached this stage indicates that it has shown promising results in earlier stages of development.
It is worth noting that Luminespib has reached Phase 2 in both global and Chinese markets. This suggests that the drug has gained recognition and interest not only internationally but also within the Chinese pharmaceutical industry.
In terms of regulation, Luminespib is classified under the Special Review Project. This classification implies that the drug may have unique characteristics or potential benefits that warrant special attention from regulatory authorities.
In summary, Luminespib is a small molecule drug developed by Ligand UK Ltd. It targets HSP90 and shows potential in treating various neoplasms, including head and neck neoplasms, adenocarcinoma of the lung, and squamous cell carcinoma. The drug has reached Phase 2 in both global and Chinese markets, indicating promising results in earlier stages of development. Luminespib falls under the Special Review Project category, suggesting unique characteristics or potential benefits that require special regulatory attention.