Sulfadiazine Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Sulfadiazine's R&D Progress
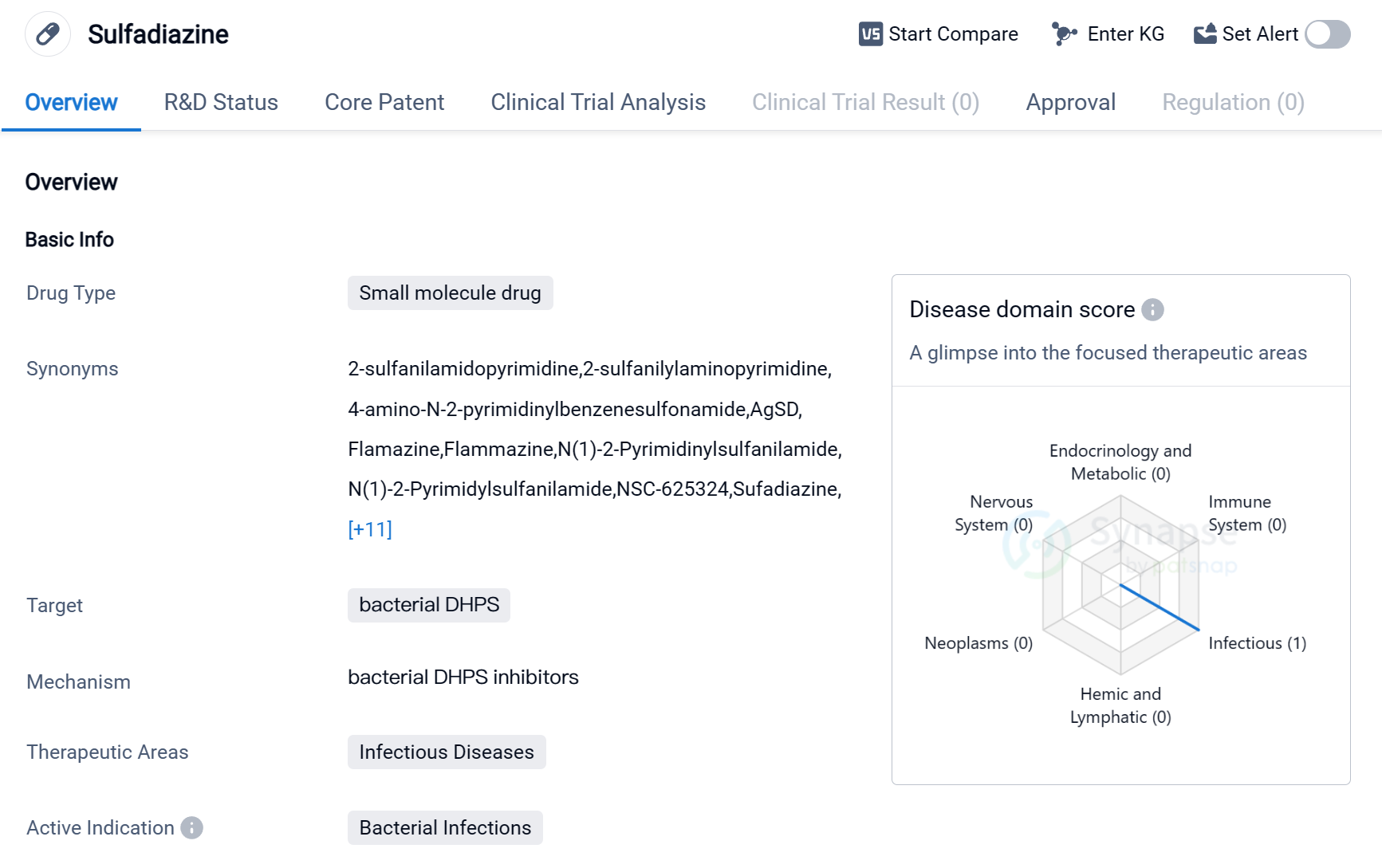
Sulfadiazine is a small molecule drug that falls under the category of biomedicine. It is primarily used to target bacterial dihydropteroate synthase (DHPS), making it effective against bacterial infections. The drug is mainly utilized in the therapeutic area of infectious diseases, where it has shown promising results.
Sulfadiazine has received approval for use globally, indicating its widespread acceptance and recognition.
The first approval of Sulfadiazine took place in the United States in August 1941, making it a well-established drug with a long history of use. This early approval demonstrates the drug's effectiveness and its ability to address the medical needs of patients suffering from bacterial infections.
As a small molecule drug, Sulfadiazine offers several advantages. Its small size allows for easy absorption and distribution within the body, enhancing its therapeutic potential. Additionally, small molecule drugs often have well-defined chemical structures, making them easier to manufacture and control for quality.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sulfadiazine: Bacterial DHPS inhibitor
Bacterial DHPS inhibitors are a type of drugs that target the enzyme dihydropteroate synthase (DHPS) in bacteria. DHPS is an essential enzyme involved in the synthesis of folate, which is necessary for the production of DNA and other important cellular processes in bacteria. By inhibiting DHPS, these drugs prevent the bacteria from synthesizing folate, leading to the disruption of essential cellular functions and ultimately killing the bacteria. Bacterial DHPS inhibitors are commonly used in the treatment of bacterial infections caused by susceptible strains of bacteria. They are particularly effective against certain types of bacteria, such as those responsible for urinary tract infections and respiratory tract infections. These inhibitors are an important class of antibiotics and play a crucial role in combating bacterial infections.
Drug Target R&D Trends for Sulfadiazine
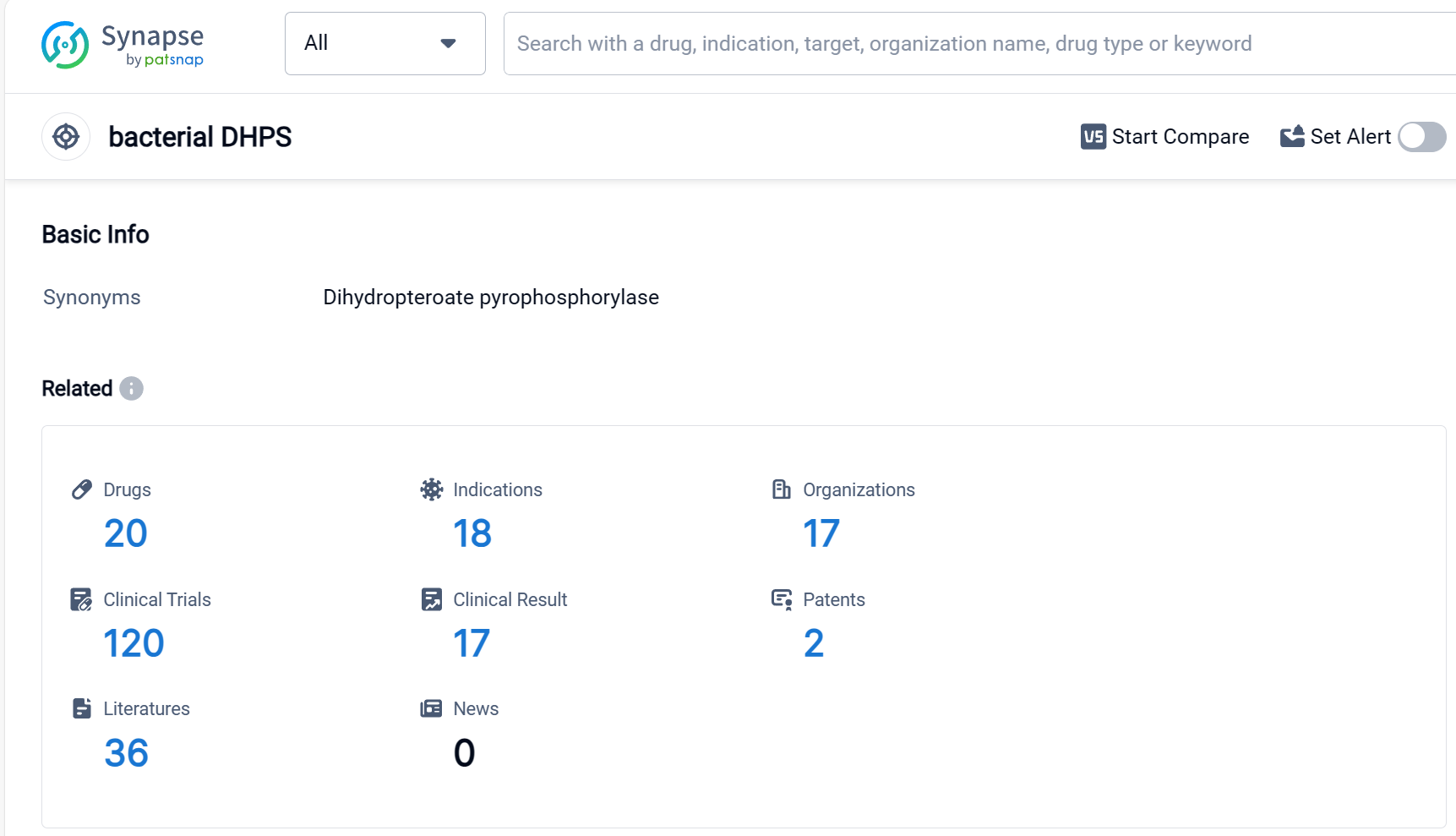
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 20 bacterial DHPS drugs worldwide, from 17 organizations, covering 18 indications, and conducting 120 clinical trials.
Based on the analysis of the provided data, Pfizer Inc. is the company with the highest number of drugs in the approved phase targeting bacterial DHPS. The most common indication for these drugs is bacterial infections. Small molecule drugs are progressing rapidly in the development of treatments for bacterial DHPS. China is leading in the development of drugs targeting bacterial DHPS, followed by the United States. The competitive landscape for target bacterial DHPS is diverse, with multiple companies and countries/locations involved in the development of drugs. The future development of target bacterial DHPS will likely continue to focus on small molecule drugs and expand to other indications related to bacterial infections.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Sulfadiazine is a small molecule drug that targets bacterial DHPS and is primarily used in the treatment of infectious diseases. It has received approval globally, with its first approval dating back to 1941 in the United States. The drug's long history and widespread acceptance highlight its effectiveness in combating bacterial infections. As a small molecule drug, Sulfadiazine offers advantages such as easy absorption and distribution within the body, as well as simplified manufacturing processes.