Understanding Plasminogen activators Inhibitors and Methods to Keep Abreast of Their Recent Developments
Plasminogen activators are enzymes that play a crucial role in the human body's fibrinolytic system. They are responsible for converting plasminogen, an inactive precursor, into plasmin, an active enzyme that breaks down blood clots. Plasminogen activators are essential in maintaining the balance between clot formation and dissolution, preventing excessive clotting and ensuring proper blood flow. They are used therapeutically in the treatment of conditions such as acute ischemic stroke, deep vein thrombosis, and pulmonary embolism, where rapid clot dissolution is necessary. Plasminogen activators, such as tissue plasminogen activator (tPA), are administered intravenously to restore blood flow and prevent tissue damage caused by blood clots.
The Plasminogen Activator Inhibitor (PAI) was first discovered in the plasma of pregnant women by Brakman and Astrup in 1963, and subsequently identified by other researchers. Currently, four types of PAIs have been identified in humans: PAI-1, PAI-2, PAI-3, and PAI-4. Among these, PAI-1 accounts for 99% of the activity and plays a major role. PAI-1 is widely distributed in the body’s endothelial cells, smooth muscle cells, liver cells, fat cells, macrophages, neutrophils, glomerular mesangial cells, platelets, and some malignant tumor cells. Plasma PAI-1 is mainly produced by endothelial cells and liver cells. PAI-1 is extremely unstable both in vivo and in vitro, with a half-life of about 20 minutes. This is mainly due to the oxidation of the active center of glutamic acid and the degradation of PAI-1 by cell phagocytosis after forming a complex with tissue-type plasminogen activator (t-PA) or urokinase-type plasminogen activator (u-PA). Plasma PAI-1 mainly exists in two forms: one is active, known as activated PAI-1; the other is inactive, known as latent PAI-1. These two forms of PAI-1 have the same molecular weight and immunoreactivity, but different conformations. The active PAI-1 can be stabilized and maintain its activity by binding to vitronectin.
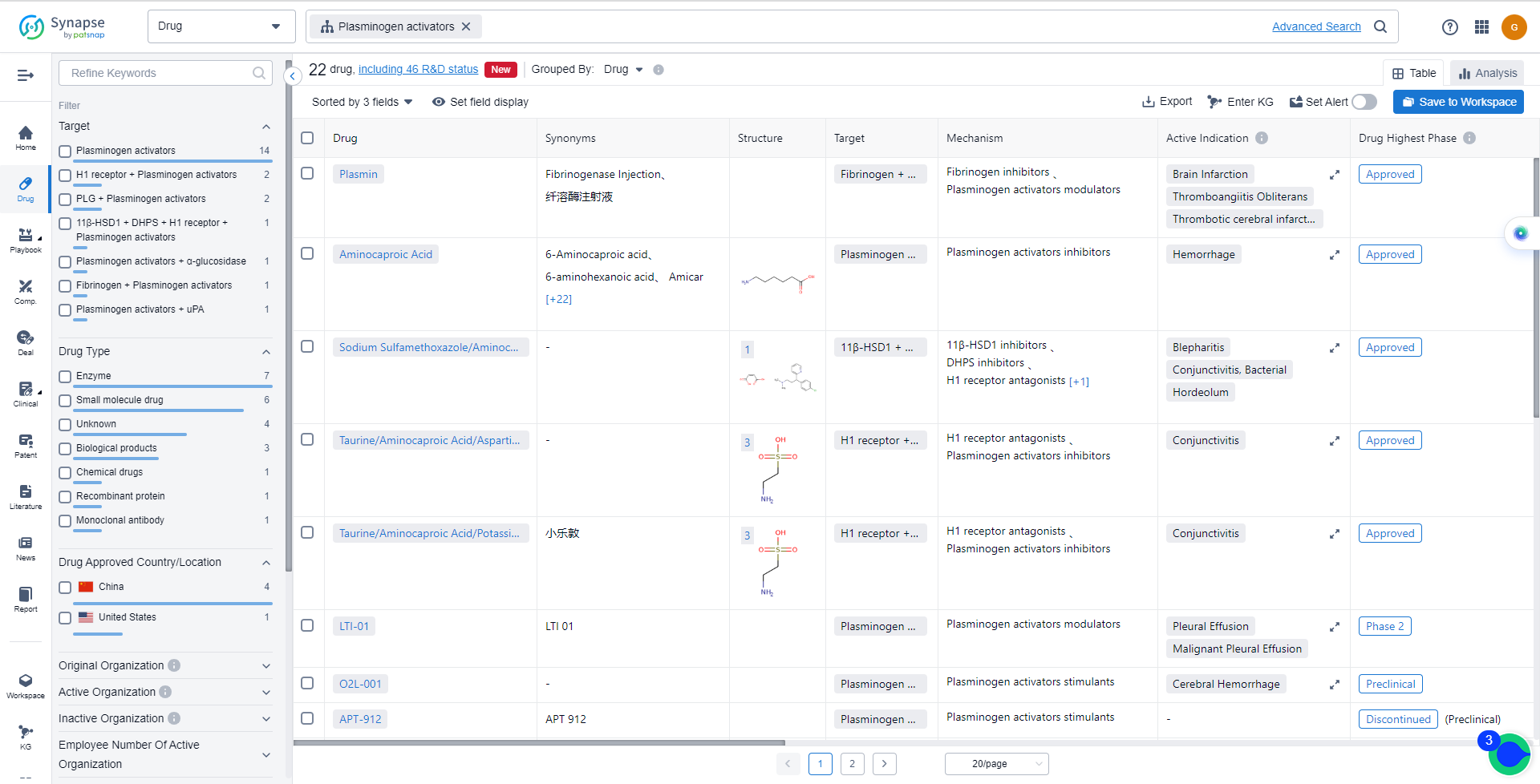
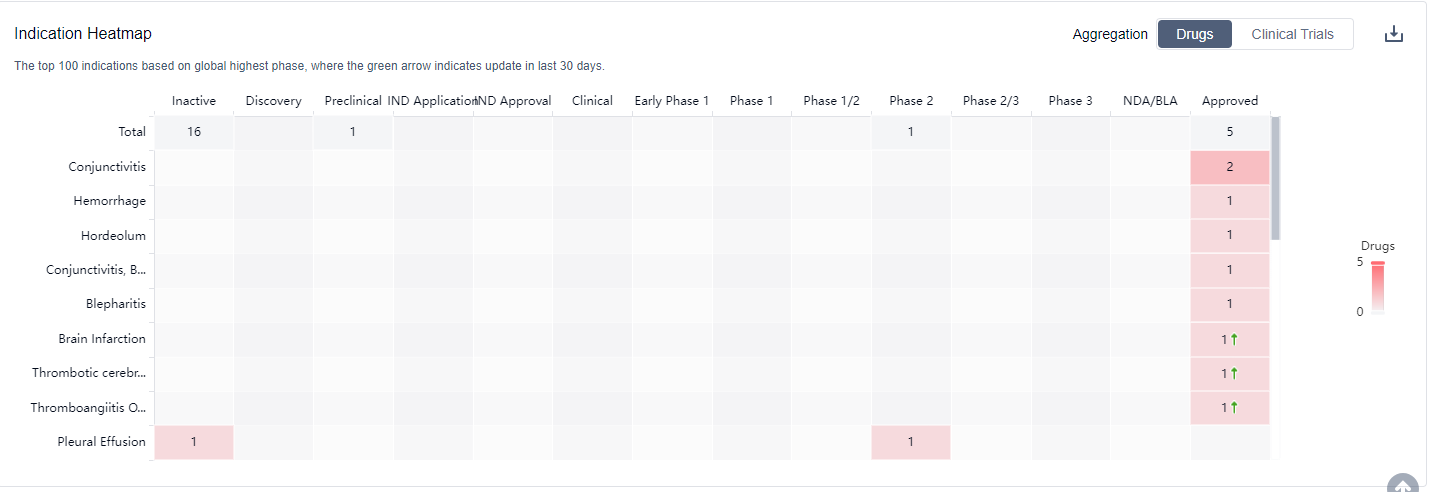
The analysis of the target Plasminogen activators reveals a competitive landscape with companies such as Hunan Er-Kang Pharmaceutical Co., Ltd., Akorn Operating Co. LLC, Hainan Sihuan Pharmaceutical Co. Ltd., and Beijing Science Sun Pharmaceutical Co., Ltd. leading the way with approved drugs. These drugs have been approved for indications such as Conjunctivitis, Hemorrhage, Hordeolum, and more. The development of small molecule drugs and enzymes is progressing rapidly, indicating potential breakthroughs in the treatment of various medical conditions. China is at the forefront of the development of Plasminogen activators, followed by the United States. Other countries/locations are also actively involved in the development of drugs for this target. Overall, the target Plasminogen activators show promise in the pharmaceutical industry, with potential for further growth and development in the future.
How do they work?
Plasminogen activators inhibitors are substances that inhibit the activity of plasminogen activators. Plasminogen activators are enzymes that convert plasminogen, an inactive precursor, into plasmin, an active enzyme involved in the breakdown of blood clots. Plasminogen activators inhibitors, as the name suggests, prevent or inhibit the activation of plasminogen, thereby reducing the breakdown of blood clots.
From a biomedical perspective, plasminogen activators inhibitors play a crucial role in regulating the clotting and fibrinolysis processes in the body. They help maintain the balance between clot formation and clot dissolution. By inhibiting plasminogen activators, these inhibitors can prevent excessive clot breakdown, which may be beneficial in certain medical conditions where clot formation is necessary, such as during surgery or in the treatment of bleeding disorders.
In the context of biomedicine, plasminogen activators inhibitors have therapeutic implications. They can be used as medications to prevent the breakdown of blood clots in conditions such as deep vein thrombosis, pulmonary embolism, or stroke. By inhibiting plasminogen activators, these inhibitors can help stabilize blood clots and prevent their dissolution, promoting clot stability and reducing the risk of complications.
It is important to note that the use of plasminogen activators inhibitors should be carefully monitored and administered under medical supervision, as their effects on the clotting system need to be balanced to avoid excessive clot formation or inhibition.
List of Plasminogen activators Inhibitors
The currently marketed Plasminogen activators inhibitors include:
- Plasmin
- Aminocaproic Acid
- Taurine/Aminocaproic Acid/Aspartic Acid/Chlorphenamine Maleate
- Taurine/Aminocaproic Acid/Potassium L-aspartate/Chlorphenamine Maleate
- LTI-01
- O2L-001
- APT-912
- BM-06021
- CVS-3083
- M-5
For more information, please click on the image below.
What are Plasminogen activators inhibitors used for?
Plasminogen activators inhibitors are used therapeutically in the treatment of conditions such as acute ischemic stroke, deep vein thrombosis, and pulmonary embolism, where rapid clot dissolution is necessary. For more information,please click on the image below to log in and search.
How to obtain the latest development progress of Plasminogen activators inhibitors?
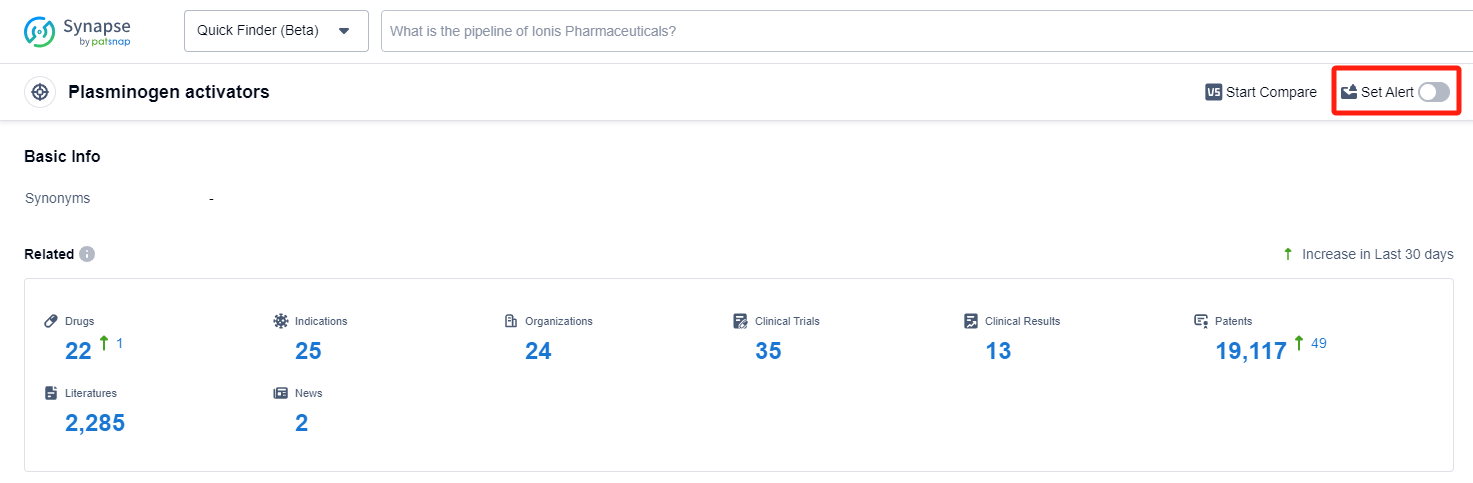
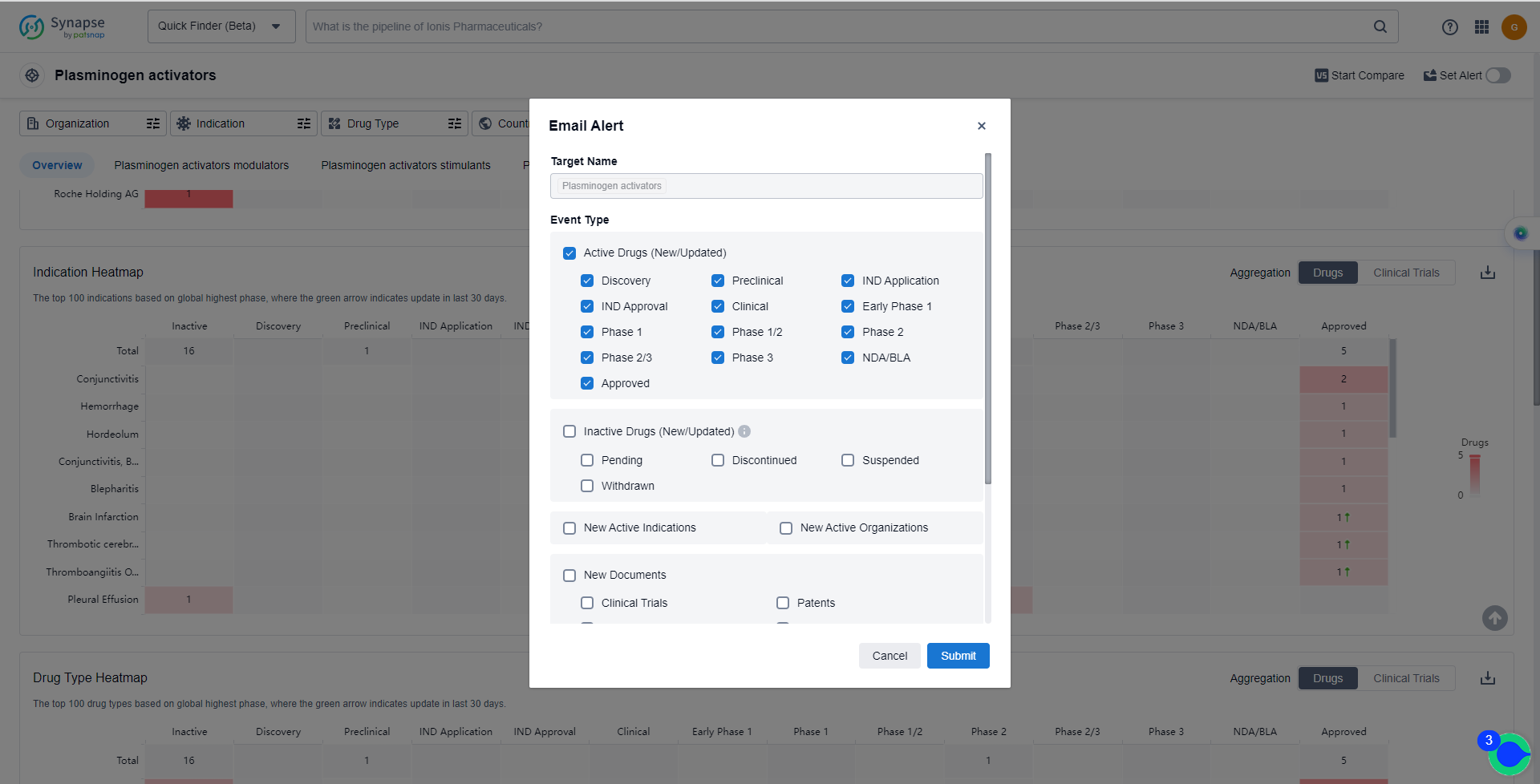
In the Synapse database, you can keep abreast of the latest research and development advances of Plasminogen activators inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!