Market Analysis of Droperidol in the USA: Regulatory History, Safety Risks, and Reintroduction Pathways
Overview
The US market has 1 drug approved for droperidol, which is currently listed with a "Withdrawn" market status. Droperidol is a small molecule drug that acts as a D2 receptor antagonist, primarily used for treating nausea and vomiting. Originally developed decades ago, the drug has faced regulatory challenges in the US market.
Detailed Description
Drug Information
Droperidol was developed by Rising Pharmaceuticals, Inc. and was approved in the USA on June 11, 1970.
| Approval Number | Approval Company | Approval Date | Dosage Form | Specification | Administration Route | Indication | Approval Status |
|---|---|---|---|---|---|---|---|
| 016796 | Rising Pharmaceuticals, Inc. | 1970-06-11 | Injection | 2.5MG/ML | Injection | Nausea and vomiting | Withdrawn |
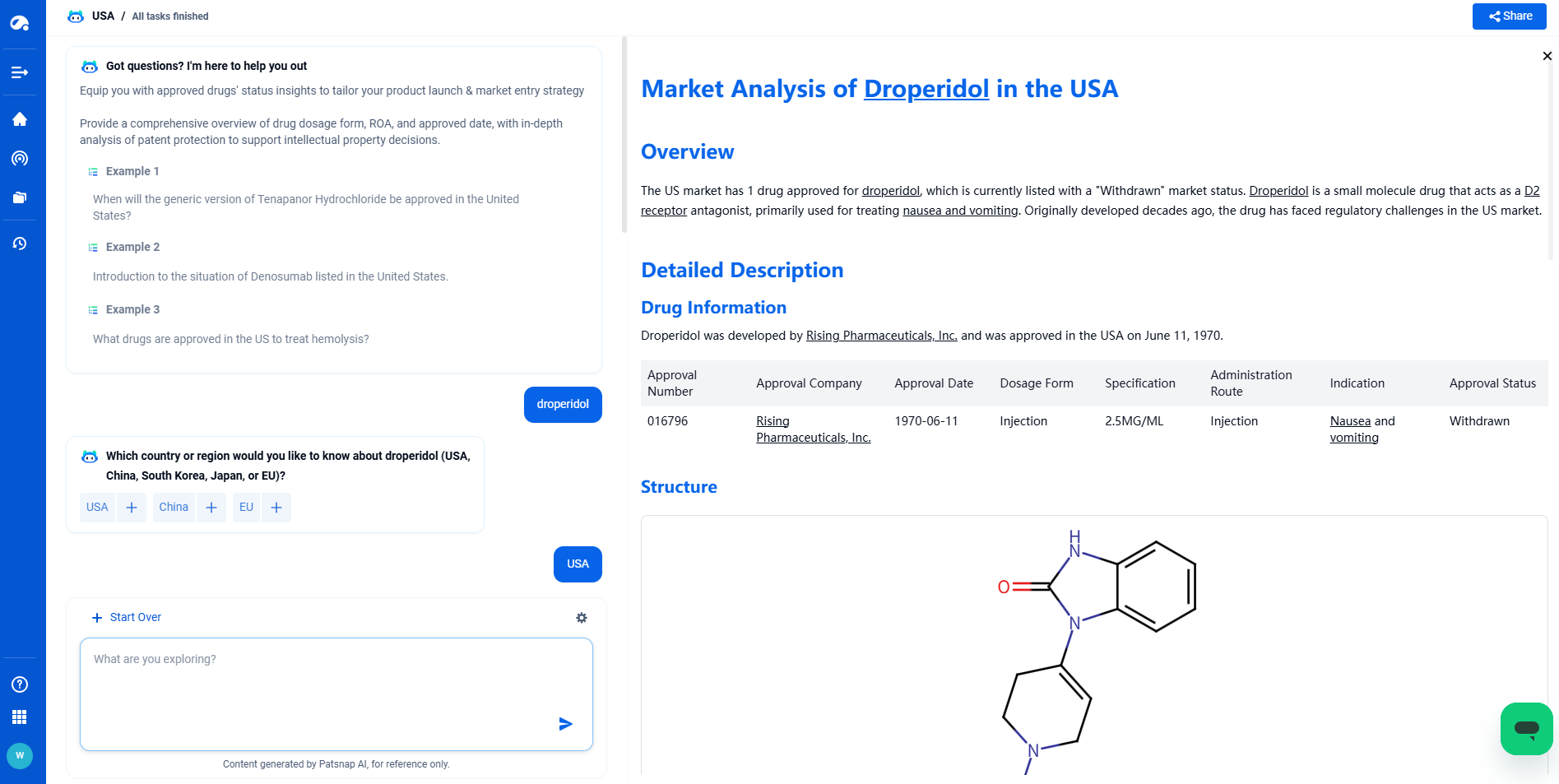
Structure
Patent Barrier Analysis
Registration Patent Analysis
No registration patents were found for droperidol in the FDA Orange Book.
Other Patent Barrier Analysis
The original compound patent for droperidol has expired, and there are no active core patents in the USA. The following patents were identified:
| Patent Number | Simple Legal Status | Application Date | Estimated Expiry | Patent Type | Applicant |
|---|---|---|---|---|---|
| US3161645A | Inactive | 1962-12-18 | 1981-12-15 | Product Compound | NV RES LAB DR C JANSSEN |
| US5981552A | Inactive | 1998-10-23 | 2018-10-23 | New Use | Akorn, Inc. |
| WO1993014757A1 | PCT designated stage expired | 1993-01-25 | - | New Use, Device, Formulation | Texas A&M University |
| CN117229255A | Active | 2023-09-08 | 2043-09-08 | Process | Shandong Chenlong Pharmaceutical Co., Ltd. |
There is one active patent related to droperidol, but it is a process patent in China rather than the USA, so it does not affect the US market.
Clinical Results
Based on FDA label clinical insights:
Cardiac Conduction Study in Surgical Patients
A controlled clinical study evaluated the effect of droperidol on the QT interval in 40 patients without known cardiac disease who underwent extracranial head and neck surgery. Patients received droperidol at three dosing levels (0.1, 0.175, and 0.25 mg/kg), demonstrating a dose-dependent prolongation of the QT interval with median QTc increases of 37, 44, and 59 msec, respectively. This experiment assessed the electrophysiological safety of droperidol in the perioperative setting.
Mutagenicity Assessment in Animal Models
Preclinical evaluations using the micronucleus test in female rats with single oral doses of droperidol as high as 160 mg/kg revealed no mutagenic effects.
Fertility Study in Rats
An oral study in rats with doses of 0.63, 2.5, and 10 mg/kg (approximately 2, 9, and 36 times the maximum recommended human IV/IM dosage, respectively) showed no impairment of fertility in either male or female rats.
Pharmacodynamic and Safety Evaluations in the Clinical Setting
Clinical evaluations noted dose-related cardiovascular events (such as hypotension and tachycardia), extrapyramidal side effects, and potential interactions with concurrently administered drugs (for example, CNS depressants or other drugs known to prolong the QT interval) .
Infringement Cases
No patent infringement incidents involving droperidol have been documented according to the available references.
Policy and Regulatory Risk Warning
After a comprehensive search, droperidol has no market exclusivity or data protection period in the USA. However, it should be noted that the drug's market status is listed as "Withdrawn," which suggests regulatory concerns. In December 2001, the FDA issued a black box warning about the risk of QT prolongation and potential for fatal cardiac arrhythmias, which significantly impacted the drug's use in the US market.
Market Entry Assessment & Recommendations
Regulatory Strategy: Any company considering reintroducing droperidol to the US market must address the safety concerns that led to its withdrawal, particularly the QT prolongation issue. Developing a comprehensive Risk Evaluation and Mitigation Strategy (REMS) would be essential.
Clinical Development: Consider conducting new clinical trials with improved safety monitoring protocols to demonstrate the drug's safety profile when used appropriately. Focus on specific indications where the benefit-risk ratio is most favorable.
Formulation Improvements: Develop new formulations or delivery systems that might reduce the cardiac risks associated with droperidol while maintaining efficacy.
Market Positioning: Position any new droperidol product for specific niches where alternatives are limited or where its risk-benefit profile is most favorable, such as treatment-resistant cases of postoperative nausea and vomiting.
Educational Programs: Develop comprehensive healthcare provider education programs about appropriate patient selection, dosing, and monitoring to minimize risks.
Patent Strategy: With no active US patents creating barriers to entry, focus on developing new patentable formulations, combinations, or delivery systems to secure market exclusivity.
Competitive Analysis: Conduct a thorough evaluation of currently available antiemetics to identify specific advantages droperidol might offer over existing options.
For more scientific and detailed information of droperidol, try PatSnap Eureka Pharma CI Explorer.