EBGLYSS® (lebrikizumab) received European Commission approval for treating moderate-to-severe atopic dermatitis
Almirall S.A., an internationally recognised biopharmaceutical firm specialising in medicinal dermatology, has received approval from the EC for the usage of EBGLYSS (lebrikizumab) in the therapy of adult and teenage patients suffering from medium to acute atopic dermatitis, who are suitable for systemic treatment. The firm plans on initial market launch of the product in Germany and anticipates to extend the launch in more European nations during 2024.
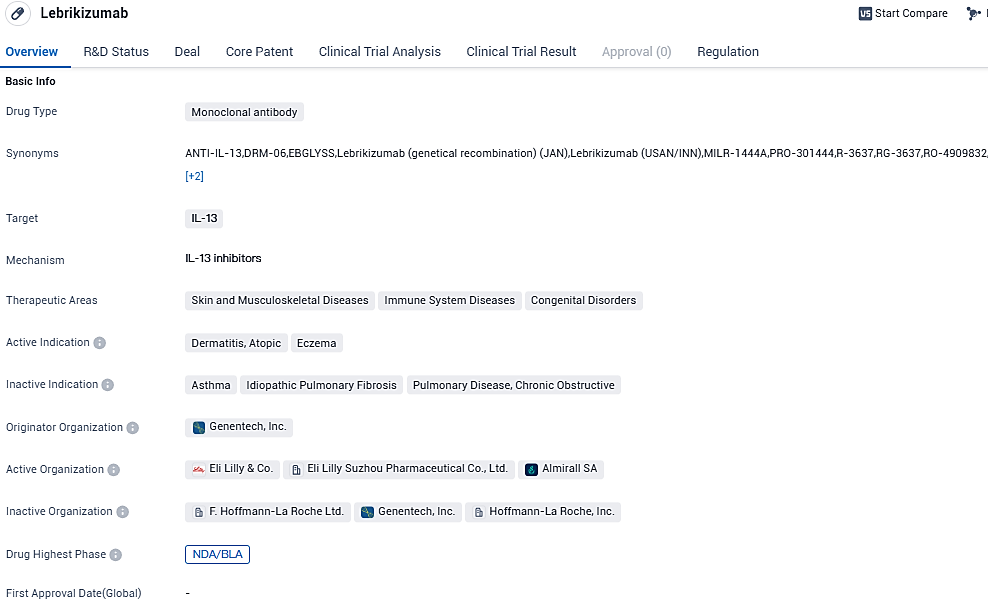
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Lebrikizumab, a monoclonal antibody, interacts powerfully with IL-13 and purposefully forestalls the development of the IL-13Rα1/IL-4Rα heterodimer complex and subsequent signals, subsequently halting the biological impact of IL-13. This significant cytokine, IL-13, plays a central role in atopic dermatitis, instigating a type-2 inflammatory cycle in the skin, resulting in skin barrier malfunction, itching, skin thickening, and infection.
Lebrikizumab embodies a substantial advancement for patients suffering from moderate-to-extreme AD who have inadequate control with topical therapy, thanks to its exclusive mode of action, established short and long-term efficacy, and safety verified for up to 2 years. Additionally, it offers a monthly maintenance dose for all patients.
Following its approval by the EC, lebrikizumab provides another essential treatment alternative for individuals grappling with moderate-to-severe AD. "This regulatory achievement underscores Almirall’s devotion to evolving innovative therapies that can significantly enrich the lives of people afflicted with skin diseases," affirmed Dr. Volker Koscielny, Almirall's Chief Medical Officer.
Almirall possesses the licensing rights for the development and commercialization of lebrikizumab for various dermatological conditions, including atopic dermatitis, throughout Europe. Eli Lilly and Company retains exclusivity for the product's growth and dissemination in the United States as well as other nations excluding Europe. Further regulatory verdicts for lebrikizumab in moderate-to-severe atopic dermatitis from additional European territories, including the UK and Switzerland, are anticipated by Almirall.
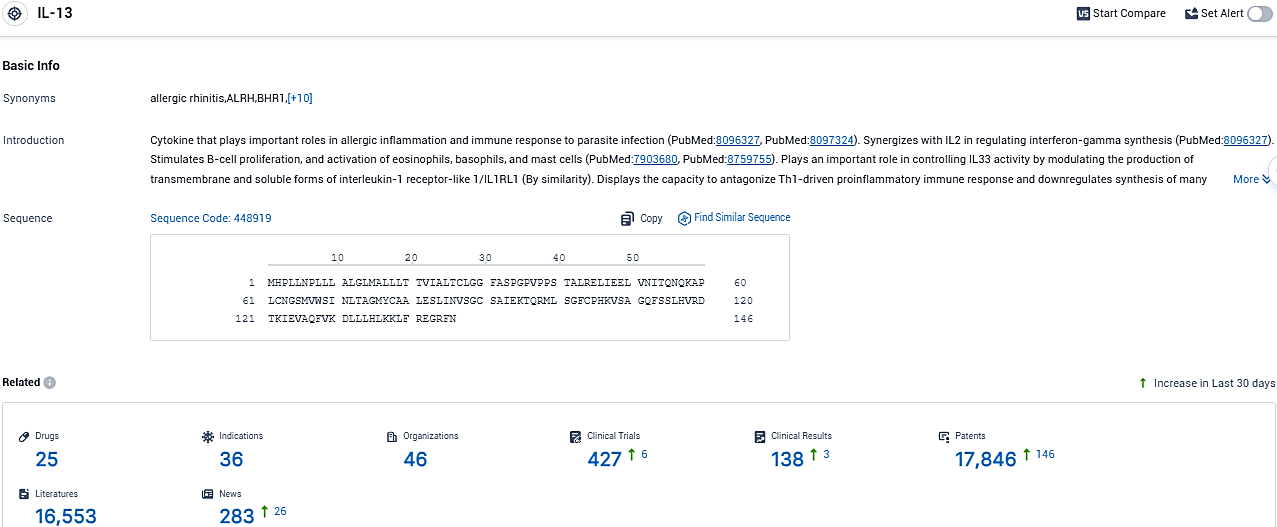
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 23, 2023, there are 25 investigational drugs for the IL13 target, including 36 indications, 46 R&D institutions involved, with related clinical trials reaching 427, and as many as 17846 patents.
Lebrikizumab targets IL-13 and is primarily used in the treatment of dermatitis, atopic eczema, and other related conditions. The drug has reached the highest phase of development globally, indicating its advanced stage of clinical trials and readiness for regulatory approval. In China, Lebrikizumab is in the early stages of development, with an IND application submitted. The drug is regulated under the Fast Track designation, highlighting its potential to address unmet medical needs efficiently.