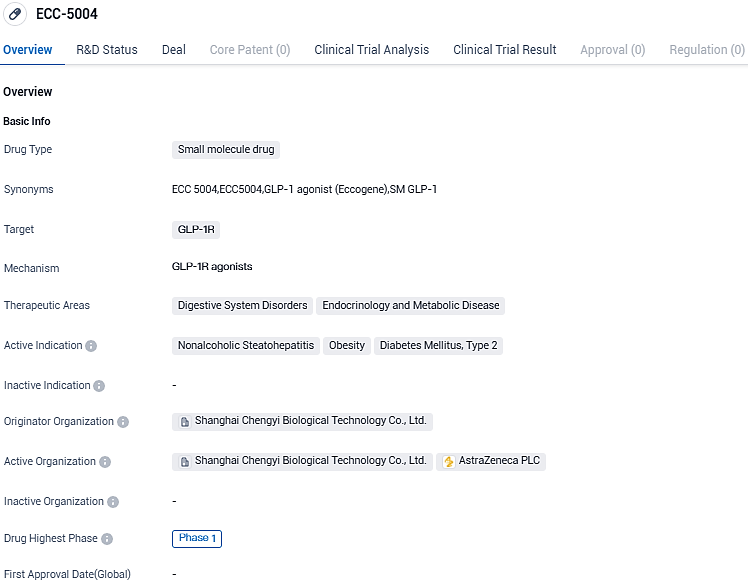
Eccogene obtains exclusive license from AstraZeneca to develop and sell ECC5004, a GLP-1 receptor agonist for cardio-metabolic diseases
Eccogene publicized that it has procured an exclusive licensure deal with AstraZeneca. According to the agreement, AstraZeneca will direct the development and commercialization phases of Eccogene's small molecular GLP-1 receptor agonist (GLP-1RA) ECC5004. This potential treatment targets obesity, type-2 diabetes, and other coexisting conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"GLP-1RA is a crucial category of medications for numerous cardiometabolic conditions. At present, there is no approved oral form of GLP-1RA. However, small molecule GLP-1RA's like ECC5004 may provide certain advantages in terms of convenience and ease of use over the current GLP-1RA treatment methods." stated Jingye Zhou, Eccogene's Chief Executive Officer.
"Given AstraZeneca's remarkable worldwide proficiency in clinical development and commercialization, the essential partnership between Eccogene and AstraZeneca will greatly expedite the development of ECC5004. This once daily, low-dose, oral small molecule GLP-1RA could potentially assist millions of global patients with these diseases." Zhou further commented.
Sharon Barr, AstraZeneca's Executive Vice President of Biopharmaceuticals R&D, expressed that "our belief is that this oral form of GLP-1RA could provide an alternative to current injectable treatments as both a likely standalone therapy as well as in combination for diseases such as type-2 diabetes, and likewise for obesity. The addition of ECC5004 to our portfolio enhances our current pipeline focused on incretin and non-incretin pathways, including our GLP-1/glucagon dual agonist and lasting amylin analogue."
According to the partnership agreement, Eccogene will initially be granted a payment of $185 million. Furthermore, Eccogene could potentially receive an extra $1.825 billion in future clinical, regulatory and commercial milestones. On top of this, Eccogene will also be entitled to tiered royalties from net product sales.
In return for this, AstraZeneca acquires exclusive rights to develop and commercialize Eccogene's GLP-1RA, ECC5004, for any potential indication in all regions apart from China. Both Eccogene and AstraZeneca will collaborate on the co-development and co-commercialization of ECC5004 in China.
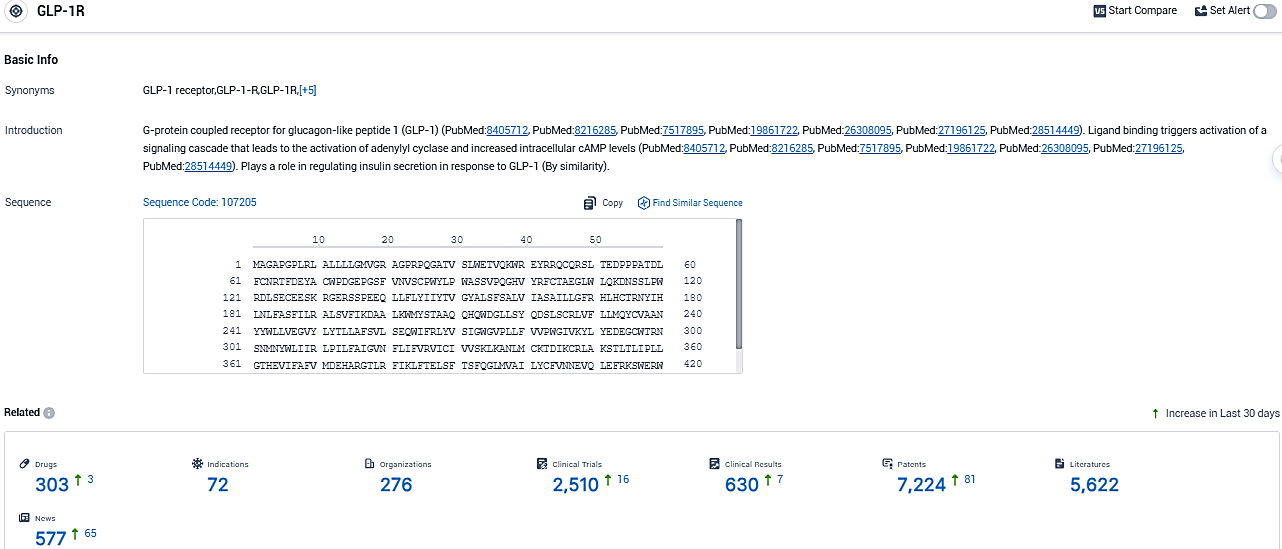
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 16, 2023, there are 303 investigational drugs for the GLP-1R and integrin target, including 72 indications, 276 R&D institutions involved, with related clinical trials reaching 2510, and as many as 7224 patents.
ECC5004 is a once daily, low dose, small molecule GLP-1RA which is currently in a US Phase I clinical trial in healthy participants and patients with type 2 diabetes. ECC5004 has been demonstrated in preclinical studies to possess desirable efficacy and safety profiles.