Efgartigimod—a revolutionary breakthrough in the treatment of myasthenia gravis
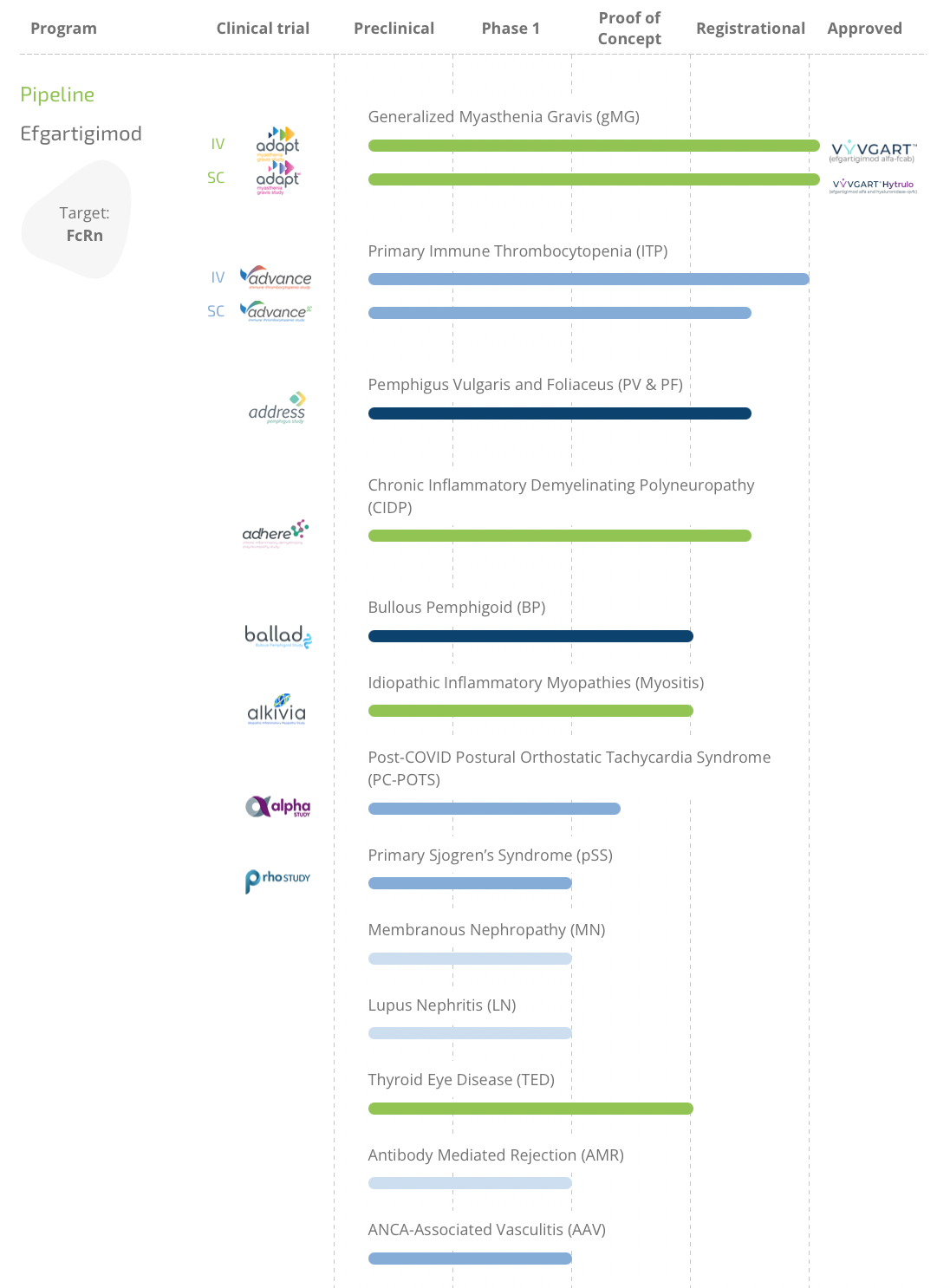
Efgartigimod/Hyaluronidase hypodermic injection, with the trade name of VYVGART HYTRULO, is a coformulation targeting FcRn and containing efgartigimod alfa and hyaluronidase. The drug was modified by Argenx BV on the basis of its company's product efgartigimod alfa-fcab and added hyaluronidase, which can enhance the permeability of subcutaneous tissue, and then changed to a subcutaneous injection preparation. On June 20, 2023, it was approved by the Food and Drug Administration (FDA) to treat adult patients with generalized myasthenia gravis (gMG) with positive Acetylcholine receptor (AChR) antibodies.
In addition to the approved Myasthenia gravis, the current research focus of the drug is to evaluate the efficacy of the drug in patients with severe autoimmune diseases. The research on primary immune thrombocytopenia (ITP), pemphigus vulgaris, Pemphigus foliaceus and defoliaceus (PV&PF), CIDP has been relatively mature and is expected to be approved in the future.
VYVGART® Hytrulo is first FDA-approved subcutaneous (SC) injectable for generalized myasthenia gravis (gMG).
Mechanism of Action
Efgartigimod alfa is a human IgG1 antibody fragment that binds to the neonatal Fc receptor (FcRn), resulting in the reduction of circulating IgG. Hyaluronidase increases permeability of the subcutaneous tissue by depolymerizing hyaluronan. This effect is transient and permeability of the subcutaneous tissue is restored within 24 to 48 hours.
Efficacy
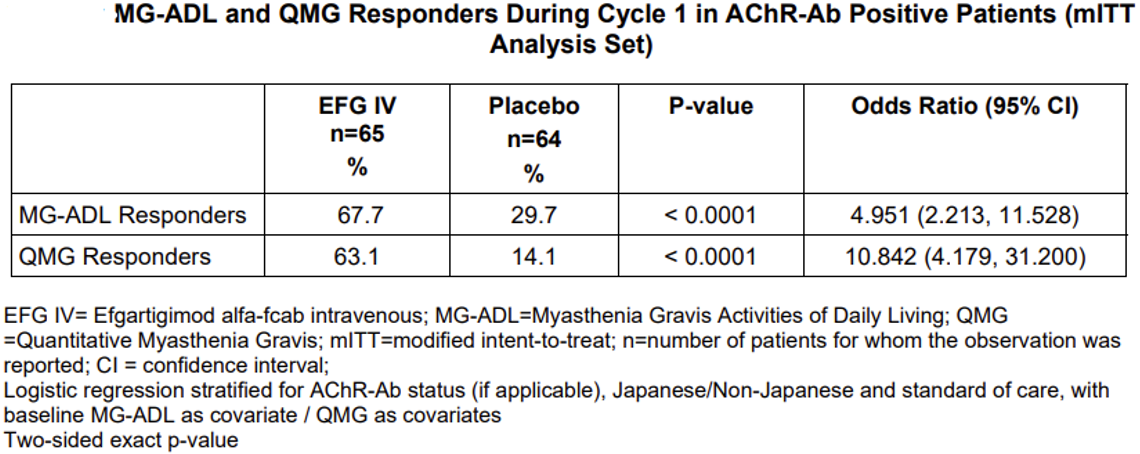
The pharmacological effect of VYVGART HYTRULO administered subcutaneously (SC) at 1,008 mg / 11,200 Units was compared to efgartigimod alfa-fcab administered intravenously at 10 mg/kg (EFG IV) in gMG patients. The maximum mean reduction in AChR-Ab level was observed at week 4, with a mean reduction of 62.2% and 59.7% in the VYVGART HYTRULO SC and efgartigimod alfa-fcab IV arm, respectively. The decrease in total IgG levels followed a similar pattern. The 90% confidence intervals for the geometric mean ratios of AChR-Ab reduction at day 29 and AUEC0-4w (area under the effect-time curve from time 0 to 4 weeks post dose) were within the range of 80% to 125%, indicating no clinically significant difference between the two formulations. In NCT03669588, the primary efficacy endpoint was the comparison of the percentage of MG-ADL responders during the first treatment cycle between treatment groups in the AChR-Ab positive population. A statistically significant difference favoring EFG IV was observed in the MG-ADL responder rate during the first treatment cycle [67.7% in the EFG IV-treated group vs 29.7% in the placebo treated group (p <0.0001)]. The most common adverse reactions (reported in at least 10% of efgartigimod alfa-fcab-treated patients) were respiratory tract infection, headache, and urinary tract infection.
Competitive Landscape
According to “Synapse”, there are a total of 12 FcRn targeted drugs, of which 3 have already been approved. In general, the competitive environment is not severe. For more information, please click on the image link below.