EicOsis Begins Phase 1b Study for EC5026 in Clinical Settings
EicOsis Human Health, a biopharmaceutical enterprise focused on pioneering a unique class of pain relievers that are non-addictive and taken orally, targeting the inhibition of the soluble epoxide hydrolase enzyme, has declared the progression of its human clinical research program. This advancement is marked by the commencement of a Phase 1b clinical trial that involves administering increasing doses to evaluate the safety profile of its investigational drug, EC5026.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.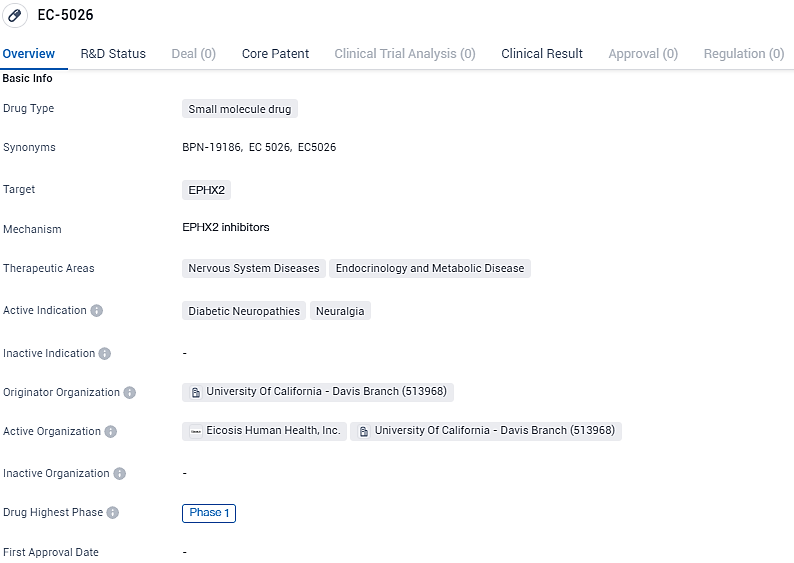
A placebo-controlled, double-masked Phase 1b clinical trial has been set up to examine the tolerability and drug disposition following daily administration of either EC5026 or a placebo for a period of one week. Early findings indicate stability in vital signs, no modifications to behavioral patterns, and a lack of serious side effects across all participants.
EC5026 is a novel therapeutic compound that operates as a potent, selectively-targeted inhibitor of the soluble epoxide hydrolase (sEH) enzyme. This enzyme has a pivotal function in managing the metabolism of bioactive lipids, which are crucial in mitigating inflammatory responses and other stress reactions stemming from injury or illness.
Through its inhibition of sEH, EC5026 provides analgesic effects by preserving the integrity of endogenous analgesic and anti-inflammatory lipids, paving the way for an innovative non-opioid solution for the management of moderate to severe pain conditions. Results from early-stage animal models indicate a lack of sedative or negative behavioral reactions, along with no potential for addiction.
EicOsis is actively engaged in the development of the sEH inhibitor EC5026 aimed at addressing pain and inflammation for various medical conditions. The company is on track to commence a landmark clinical study focused on pain management in April 2024, which will assess both the safety and pain-relieving qualities in individuals with spinal cord injuries who have not found adequate relief with current non-opioid pain medications. The drug has received Fast Track designation from the FDA, highlighting the critical need for alternatives to opioid analgesics that are both safe and effective.
"The launch of the Phase 1b trial is a pivotal achievement for the team at EicOsis Human Health, showcasing the commitment and hard work required to reach this stage. Proving its safety profile in the Phase 1b trials is paramount for advancing towards patient efficacy trials and delivering new, safe, and efficient therapies for severe illnesses," stated Cindy McReynolds, CEO of EicOsis.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!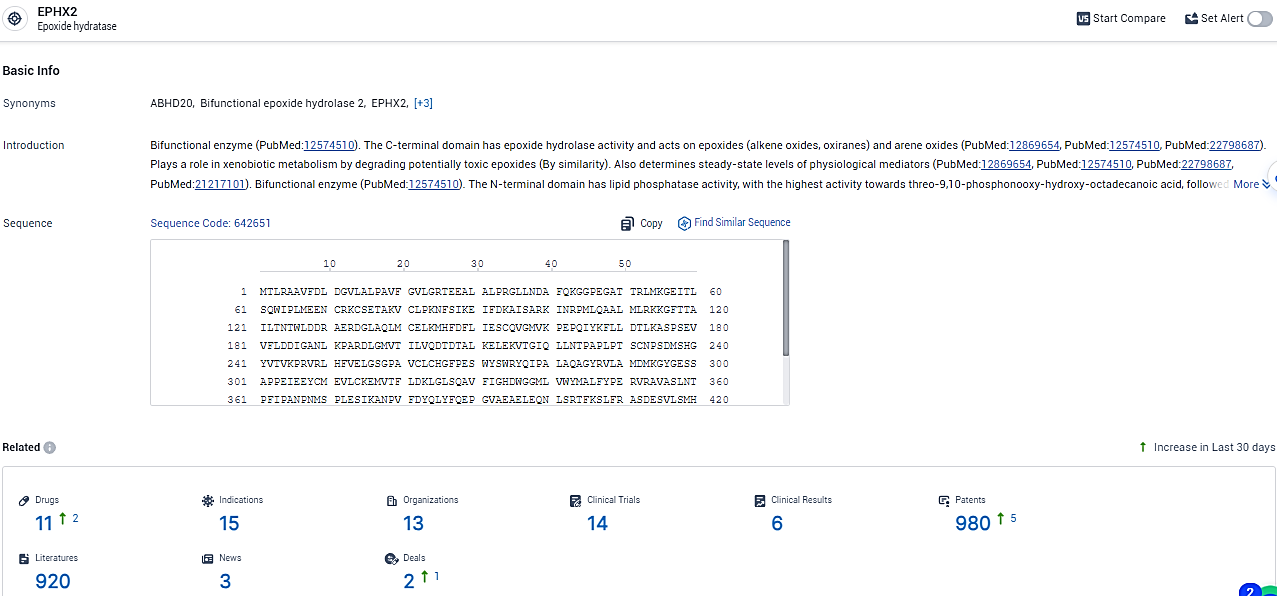
According to the data provided by the Synapse Database, As of February 6, 2024, there are 11 investigational drugs for the EPHX2 target, including 15 indications, 13 R&D institutions involved, with related clinical trials reaching 14, and as many as 980 patents.
EC-5026 is a small molecule drug being developed to target EPHX2 for the treatment of diabetic neuropathies and neuralgia. It has reached Phase 1 of clinical development and is being developed by the University of California - Davis Branch. Further research and clinical trials are needed to determine the drug's efficacy and safety profile.




