EMA Reviews GSK's Arexvy RSV Vaccine for Adults 50-59 at High Infection Risk
British pharmaceutical giant GSK plc has disclosed that its submission for authorization expansion concerning its novel adjuvanted recombinant vaccine to combat respiratory syncytial virus has been formally accepted by the European Medicines Agency. The aim is to broaden its application to encompass individuals in the 50 to 59 age group who have a heightened susceptibility to RSV infections. Should the approval be granted, this groundbreaking vaccine by GSK would stand as a pioneering preventive measure for this specific demographic.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.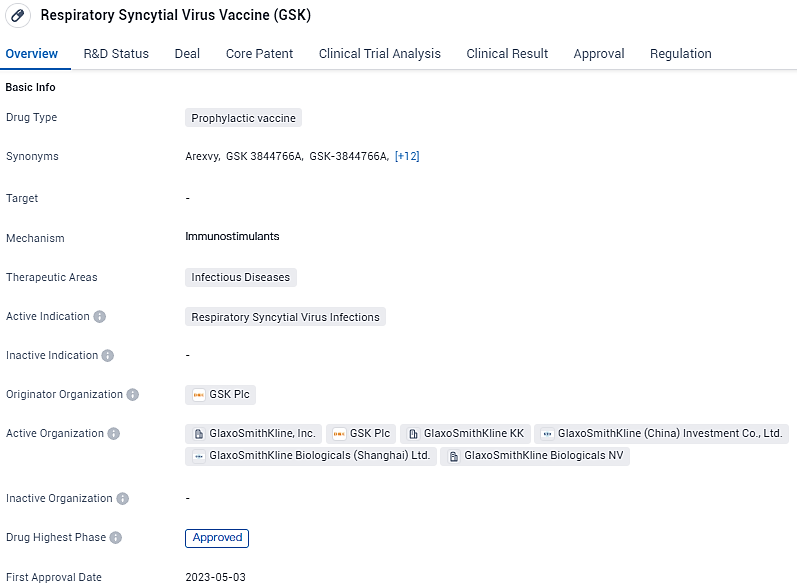
In the European market, Arexvy has received approval for preventing RSV-induced lower respiratory tract illnesses among individuals aged 60 and up. Research indicates that individuals in their fifties who are more susceptible to RSV have a similar level of risk as those aged 60 and older.
The submission for regulatory approval is a direct consequence of encouraging findings from a phase III clinical study which examined the immunogenicity and safety profile of the RSV disease
vaccine developed by GSK. This trial included participants in their fifties who faced a heightened threat of contracting RSV-LRTD, particularly due to pre-existing health concerns.
The true extent of RSV's impact on adults could be greater than currently acknowledged. This is partly because of a general lack of recognition of the disease, nonuniform diagnostic practices, and gaps in the data collection of surveillance research. Individuals suffering from health issues such as COPD, asthma, chronic heart disease, and diabetes are particularly vulnerable to RSV-related complications. In these cases, RSV can worsen the primary condition, potentially leading to severe pneumonia, hospital admissions, or even mortality.
GSK stands at the forefront among pharmaceutical firms, having submitted a request to regulatory authorities to permit the use of the RSV vaccine in adults between 50 and 59 years of age who have pre-existing medical conditions that increase their risk of RSV infection. A decision from European regulators about this application is expected to arrive in the second half of 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this indications in just a click!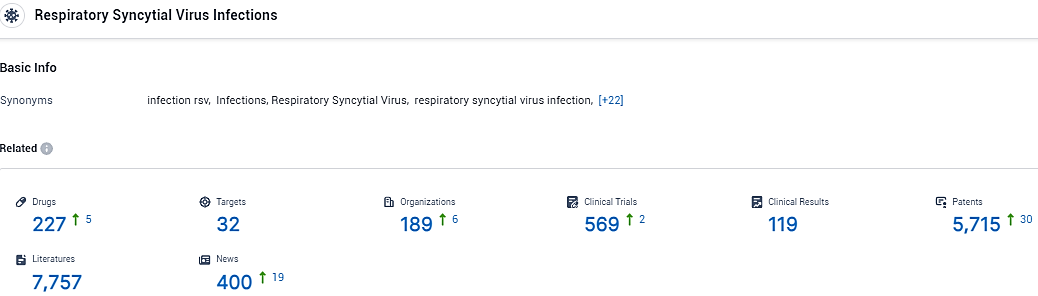
According to the data provided by the Synapse Database, As of February 6, 2024, there are 227 investigational drugs for the Respiratory Syncytial Virus Infections, including 32 targets, 189 R&D institutions involved, with related clinical trials reaching 569, and as many as 5715 patents.
the Respiratory Syncytial Virus Vaccine developed by GSK Plc is a prophylactic vaccine aimed at preventing RSV infections. It has received regulatory approval in the United States and is currently in the IND approval stage in China. The vaccine has reached the highest phase of development globally and has been granted Priority Review status, highlighting its potential to address a significant medical need.