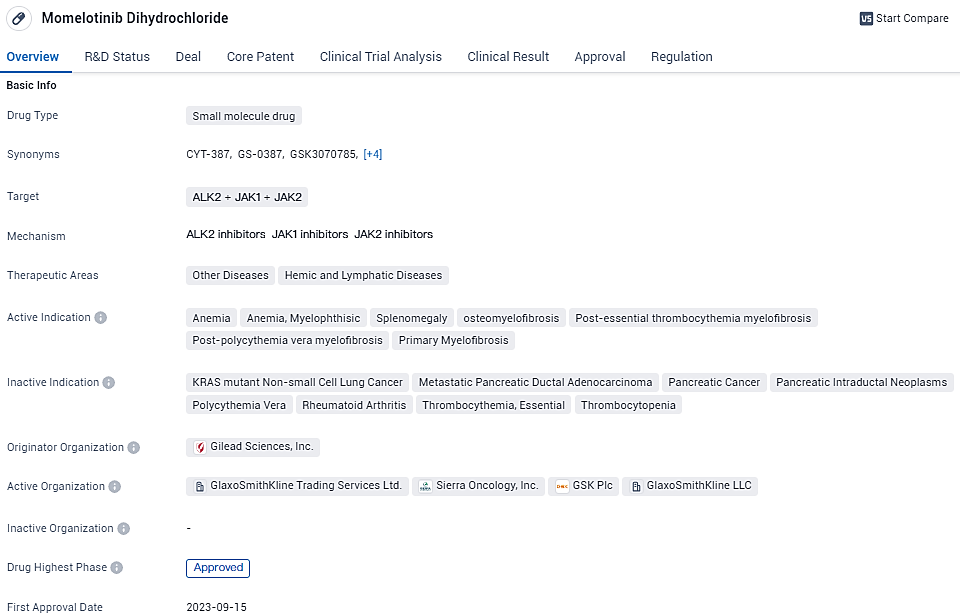
EU Commission clears GSK's drug Omjjara (momelotinib) for use
GSK plc disclosed that the European Commission has officially approved the drug Omjjara (momelotinib) for market release. This drug comes in a daily oral form and functions as an inhibitor of both JAK1/JAK2 and activin A receptor type 1. Omjjara stands as the initial treatment in the European Union to be sanctioned explicitly for adult individuals suffering from significant splenomegaly or related symptoms owing to moderate to intense anaemia conditions. This encompasses patients diagnosed with primary myelofibrosis, secondary myelofibrosis following polycythaemia vera, or myelofibrosis subsequent to essential thrombocythaemia. The approval extends to patients who either have no previous exposure to Janus kinase inhibitors or have previously been administered ruxolitinib.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Nina Mojas, who occupies the role of Senior Vice President in charge of Oncology Global Product Strategy at GSK, remarked: “Individuals coping with the condition of myelofibrosis shoulder a significant weight of symptoms, which frequently include expanded spleens, chronic tiredness, drenching sweats during the night, and discomfort in their bones. Until the recent past, specific treatments targeting these manifestations in myelofibrosis sufferers who concurrently possess low red blood cell counts were non-existent. The introduction of Omjjara in the European Union is a noteworthy development, bringing forth an innovative therapeutic approach that operates via a novel action mechanism for this patient demographic.”
The prevalence of myelofibrosis in the European Union is roughly 1 in every 10,000 people5,6. At the point of being diagnosed, it's estimated that approximately 40% of those affected have a moderate to severe deficit in red blood cells, and it's projected that almost every patient will encounter this condition as the disease progresses. Those dealing with anaemia often need more intensive supportive measures, including blood transfusions, and it's noted that over 30% cease their treatment because of the anemia. Patients dependent on transfusions typically exhibit a grim outlook with a reduction in overall lifespan.
Francesca Palandri, MD, PhD, at the IRCCS S. Orsola-Malpighi Hospital associated with Bologna University in Italy, expressed: “The EU's green light for Omjjara is a significant stride for those eligible and battling myelofibrosis, particularly impacting individuals with moderate to severe anaemic conditions who are in dire need of alternative treatments that may alleviate the effects of their ailment. The advent of a singular therapeutic intervention addressing primary aspects of myelofibrosis signifies a considerable leap for those who are eligible.”
The green-lighting of momelotinib is anchored in the MOMENTUM phase III pivotal trial outcomes, coupled with data on a subset of adult patients with significant anaemia (a haemoglobin measure under 10 g/dL) stemming from the SIMPLIFY-1 phase III trial. MOMENTUM was methodically crafted to appraise the safety profile and effective potential of momelotinib compared to danazol for treating and mitigating principal complications of myelofibrosis in a patient cohort that was anaemic, symptomatic, and had prior exposure to JAK inhibitor therapies. SIMPLIFY-1 was initiated with the intent of assessing momelotinib versus ruxolitinib concerning both safety and efficacy for myelofibrosis patients who had yet to be treated with a JAK-inhibitor.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!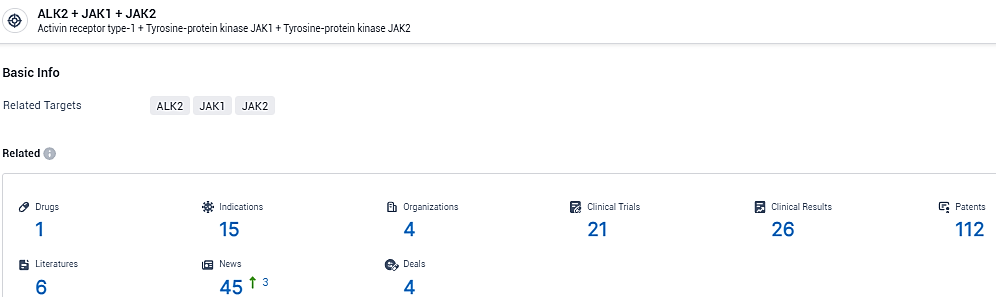
According to the data provided by the Synapse Database, As of February 5, 2024, there are 1 investigational drugs for the ALK2 and JAK1 and JAK2 target, including 15 indications, 4 R&D institutions involved, with related clinical trials reaching 21, and as many as 112 patents.
Omjjara holds promise as a small molecule drug targeting ALK2, JAK1, and JAK2, with potential applications in the treatment of various hematological disorders. Its approval in the United States and pending approval in China indicate its potential to address unmet medical needs in these regions.