Electra Therapeutics unveils initial study results from their active Phase 1b trial of ELA026 designed to combat secondary HLH
Electra Therapeutics, Inc., an enterprise in the biotech industry currently in the clinical phase, has disclosed the initial set of clinical findings for ELA026, their primary therapeutic contender engineered for tackling secondary hemophagocytic lymphohistiocytosis (sHLH), which is a perilous inflammatory condition. The organization specializes in creating innovative antibody-based treatments aimed at previously unexplored molecular targets to combat immune system diseases as well as various forms of cancer.
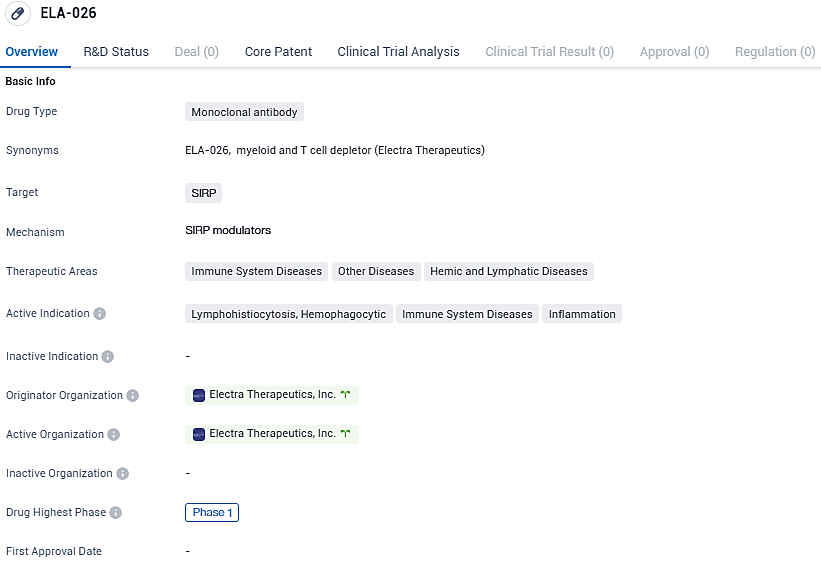
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In an ongoing Phase 1b clinical trial involving ten participants diagnosed with sHLH, initial results indicate a positive safety profile for ELA026 with the treatment achieving a 70% response rate across the cohort. Further analysis showed that 88% of the subgroup of eight evaluable patients exhibited responses. These patients, predominantly suffering from highly refractory malignancy-associated HLH, presented with unfavorable prognostic indicators and biomarkers at the onset of the study.
ELA026 operates as an immunotherapy, specifically a monoclonal antibody engineered to engage with signal regulatory protein (SIRP)-α/β1/γ on the surface of myeloid and T cells. These are the critical cells responsible for the exaggerated inflammatory response characteristic of sHLH. The aim of the current Phase 1b trial—a multi-center study which is open-label and involves multiple doses given to a single group—is to determine the safety and effectiveness of ELA026, investigate biomarkers that may indicate response, and establish an appropriate dosage to progress to Phase 2/3 trials.
Swaminathan P. Iyer, MD, a respected faculty member in the Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center, stressed the severity of sHLH, acknowledging the dire need for treatment options as none are currently approved. Dr. Iyer expressed optimism based on the observed preliminary results indicating ELA026's tolerability and therapeutic potential in the studied sHLH subset, which consists of patients with the most severe prognoses.
Representing Electra, Kim-Hien Dao, DO, PhD, the Vice President and Clinical Development lead, conveyed the company's enthusiasm about the encouraging early findings of ELA026's use in sHLH. Highlighting the successful outcomes from the initial patient groups, Dr. Dao expressed anticipation for the forthcoming stages of the study, which will continue patient enrollment and further the development of the ELA026 treatment regimen.
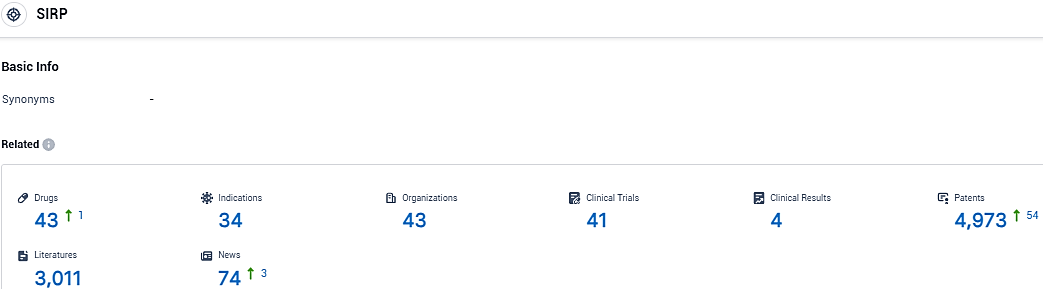
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 16, 2023, there are 43 investigational drugs for the SIRP target, including 34 indications, 43 R&D institutions involved, with related clinical trials reaching 41, and as many as 4973 patents.
By targeting SIRP, ELA-026 aims to modulate the immune response and potentially restore normal immune function. Electra Therapeutics, Inc. is the originator organization behind ELA-026. As the developer of the drug, they are responsible for conducting clinical trials and seeking regulatory approval. Phase 1 trials are typically conducted to evaluate the safety and dosage of a drug in a small group of healthy volunteers or patients. Further research and clinical trials are necessary to determine the efficacy and safety of ELA-026 in treating the indicated conditions.