Elinzanetant Significantly Reduces Menopausal Hot Flashes
Bayer is set to showcase comprehensive findings from the critical Phase III trials OASIS 1 and 2, demonstrating that the experimental drug elinzanetant significantly decreased the frequency and intensity of moderate to severe vasomotor symptoms related to menopause in comparison to a placebo.
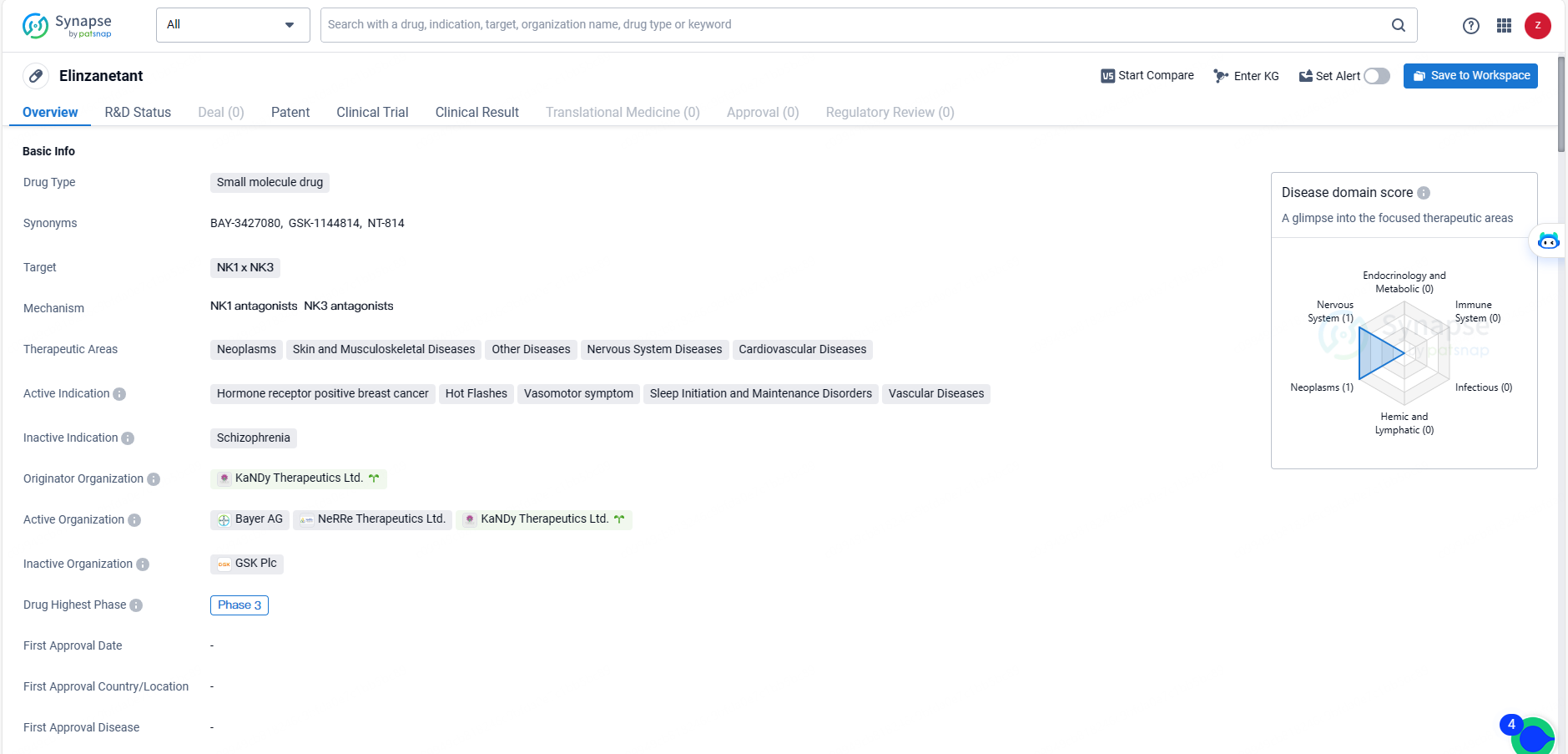
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Furthermore, elinzanetant has achieved its primary secondary endpoints by demonstrating a statistically significant decrease in VMS frequency from the baseline at week 1, along with improvements in sleep disturbances and menopause-related quality of life compared to the placebo. These findings will be showcased at the 2024 American College of Obstetricians and Gynecologists Annual Clinical & Scientific Meeting, scheduled from May 17 to 19 in San Francisco, CA, USA.
"Currently, there are limited approved non-hormonal treatments for troublesome menopausal symptoms like hot flashes and sleep disturbances. As a result, many women endure discomfort for extended periods, with most symptoms remaining untreated," said JoAnn Pinkerton, M.D., Professor and Director of Midlife Health at UVA Health. "These outcomes are promising for women dealing with moderate to severe hot flashes and bolster our confidence that elinzanetant could be a viable non-hormonal treatment option."
"The significant efficacy and positive safety profile of elinzanetant support its potential as a non-hormonal therapy for menopausal women," said Dr. Christian Rommel, member of the Executive Committee of Bayer AG’s Pharmaceutical Division and Global Head of Research and Development. "We anticipate submitting applications to health authorities for marketing approval of elinzanetant to manage moderate to severe VMS associated with menopause, enhancing our long-standing commitment to women's health."
Elinzanetant is the first dual neurokinin-1,3 (NK-1,3) receptor antagonist in late-phase clinical development for the non-hormonal management of moderate to severe VMS related to menopause, administered orally once daily.
In March 2024, Bayer disclosed favorable topline results for the third Phase III OASIS 3 study within the OASIS clinical development program, evaluating the efficacy and long-term safety of elinzanetant against placebo. The study found that elinzanetant met the primary endpoint by significantly reducing the frequency of moderate to severe vasomotor symptoms from the baseline to week 12 compared to the placebo.
The long-term safety data observed over 52 weeks in the OASIS 3 study align with previous studies and published data1,2 on elinzanetant. These findings will be presented at forthcoming scientific conferences.
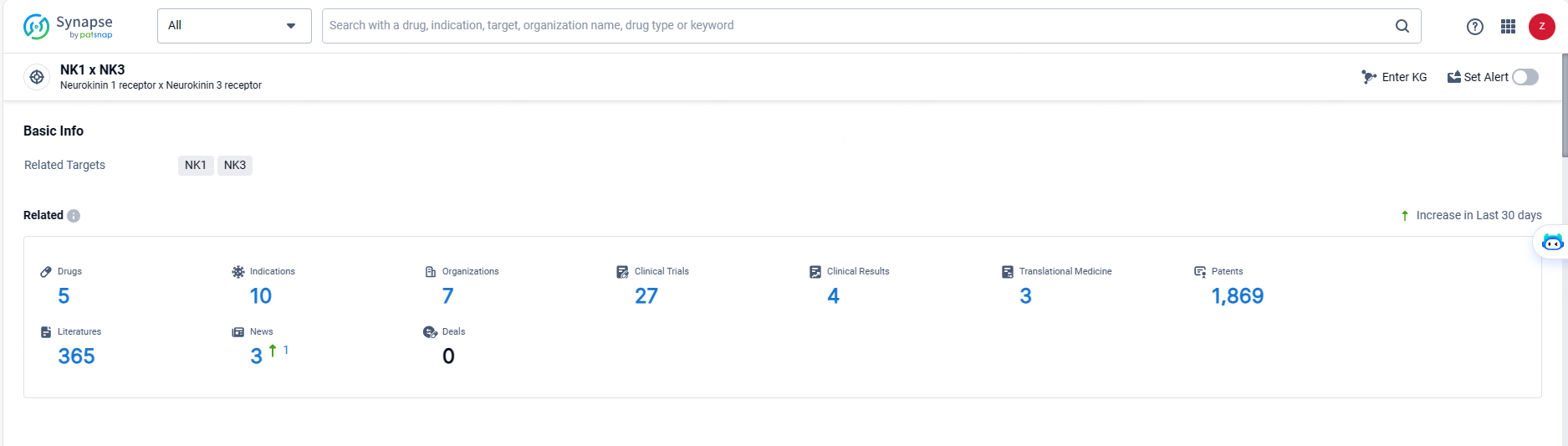
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 20, 2024, there are 5 investigational drugs for the NK-1/3 target, including 10 indications, 7 R&D institutions involved, with related clinical trials reaching 27, and as many as 1869 patents.
Elinzanetant is the first dual neurokinin-1,3 receptor antagonist, in late-stage clinical development for the non-hormonal treatment of moderate to severe VMS associated with menopause, administered orally once daily. Elinzanetant may address moderate to severe VMS by modulating a group of estrogen sensitive neurons in the hypothalamus region of the brain, which, with the decrease of estrogen, become hypertrophic and lead to a hyperactivation of the thermoregulatory pathway, consequently disrupting body heat control mechanisms resulting in VMS. Elinzanetant may also decrease sleep disturbances associated with menopause.