Incyte Announces Acquisition of Escient Pharmaceuticals, Expanding First-in-Class Drug Pipeline Focused on MRGPRX2/MRGPRX4
On April 23, global biopharmaceutical company Incyte announced the finalization of an agreement to fully acquire clinical-stage drug development company Escient Pharmaceuticals. The acquisition includes Escient's pipeline of first-in-class novel therapeutics, such as the oral antagonist pipelines EP262 and EP547 targeting Mas-related G-protein-coupled receptor X2 (MRGPRX2) and MRGPRX4, respectively. This strategic move aims to further strengthen Incyte's research and development activities in the field of inflammation and autoimmune diseases (IAI).
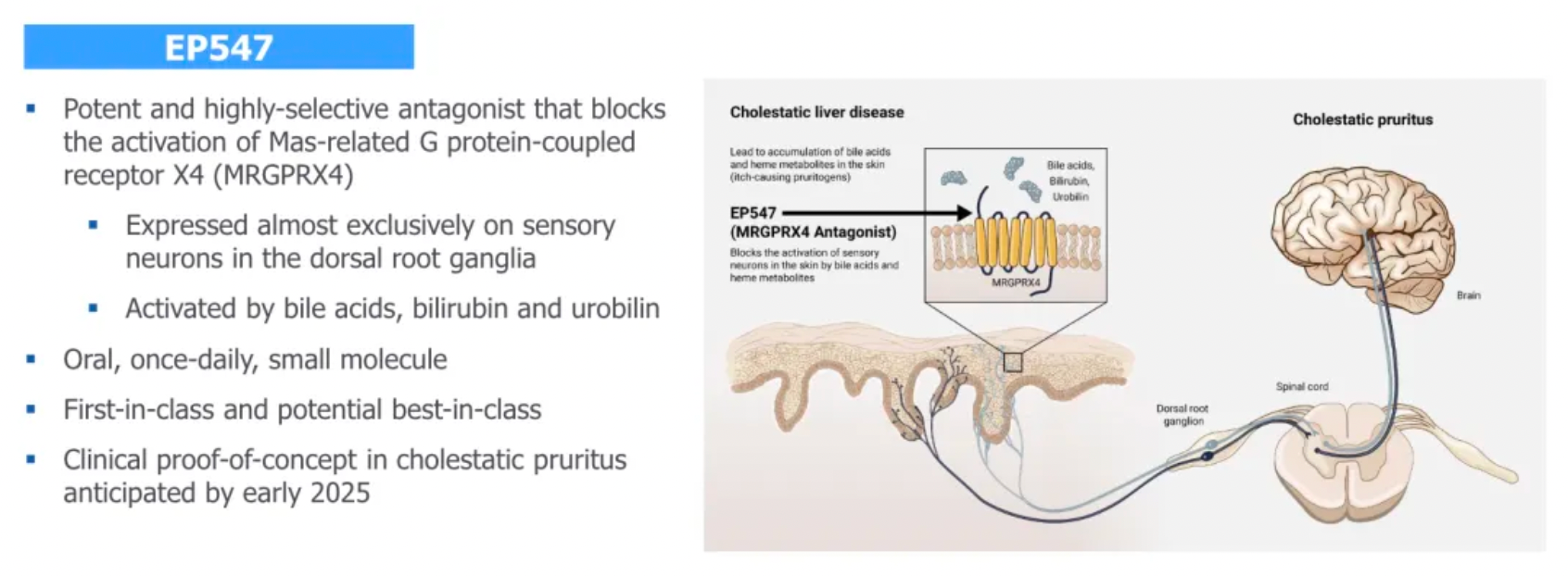
EP262 is a potentially transformative first-in-class therapeutic. As a once-daily small molecule oral antagonist, it highly selectively inhibits MRGPRX2. Its mechanism of action has been validated in chronic inducible urticaria and it is advancing into a Phase II clinical trial for chronic spontaneous urticaria. Preliminary studies suggest that EP262 can not only improve skin lesions similar to atopic dermatitis but also reduce Type 2 inflammation markers. This offers new hope for treating a variety of mast cell-mediated diseases, such as atopic dermatitis, chronic inducible urticaria, and chronic spontaneous urticaria.
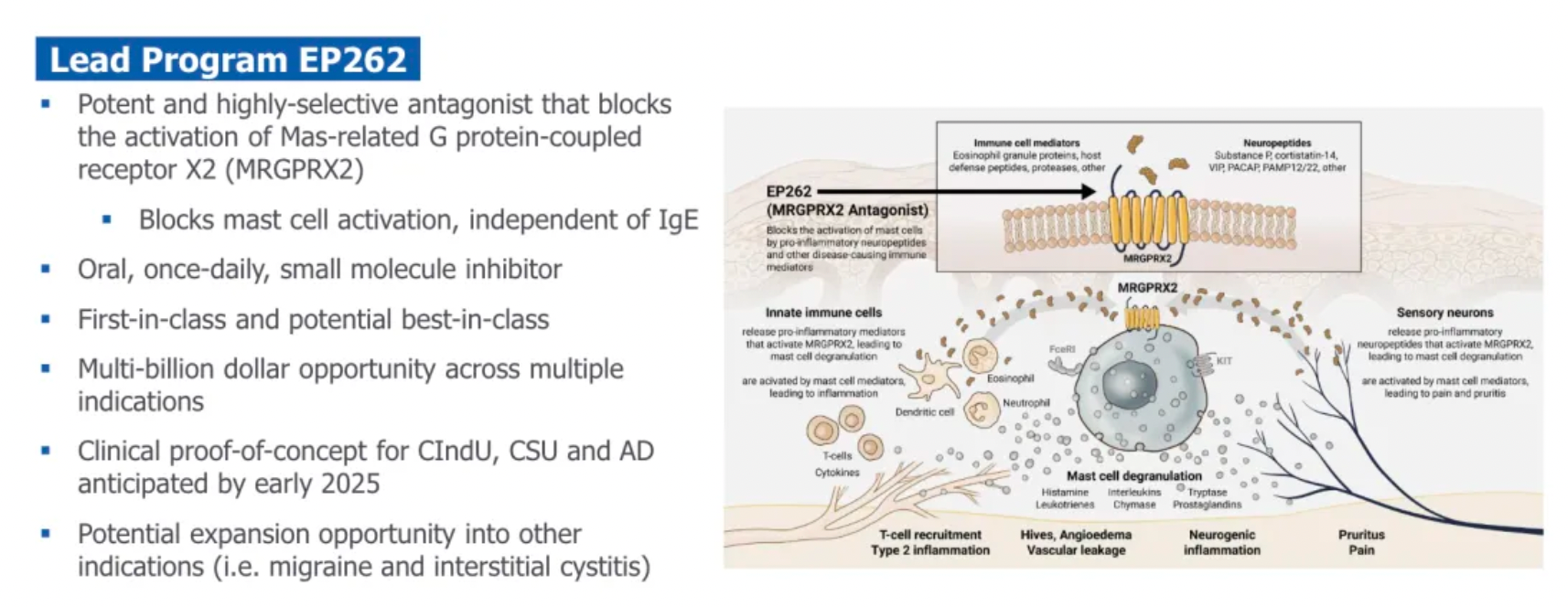
Another drug, EP547, is regarded as a first-in-class oral antagonist targeting MRGPRX4. It holds promise for treating cholestatic pruritus and other severe itching conditions, providing patients with a more precise and effective treatment option.
According to the terms of the agreement, Incyte will acquire Escient and its assets for $750 million plus Escient’s net cash at the time of closing (subject to standard adjustments). The acquisition is expected to be completed in the third quarter of 2024.
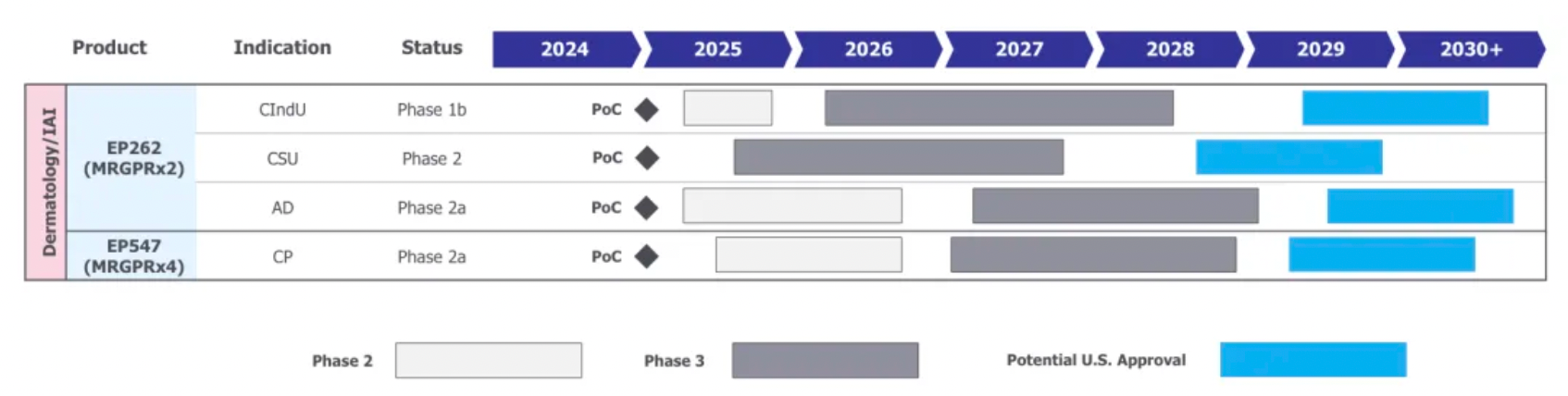
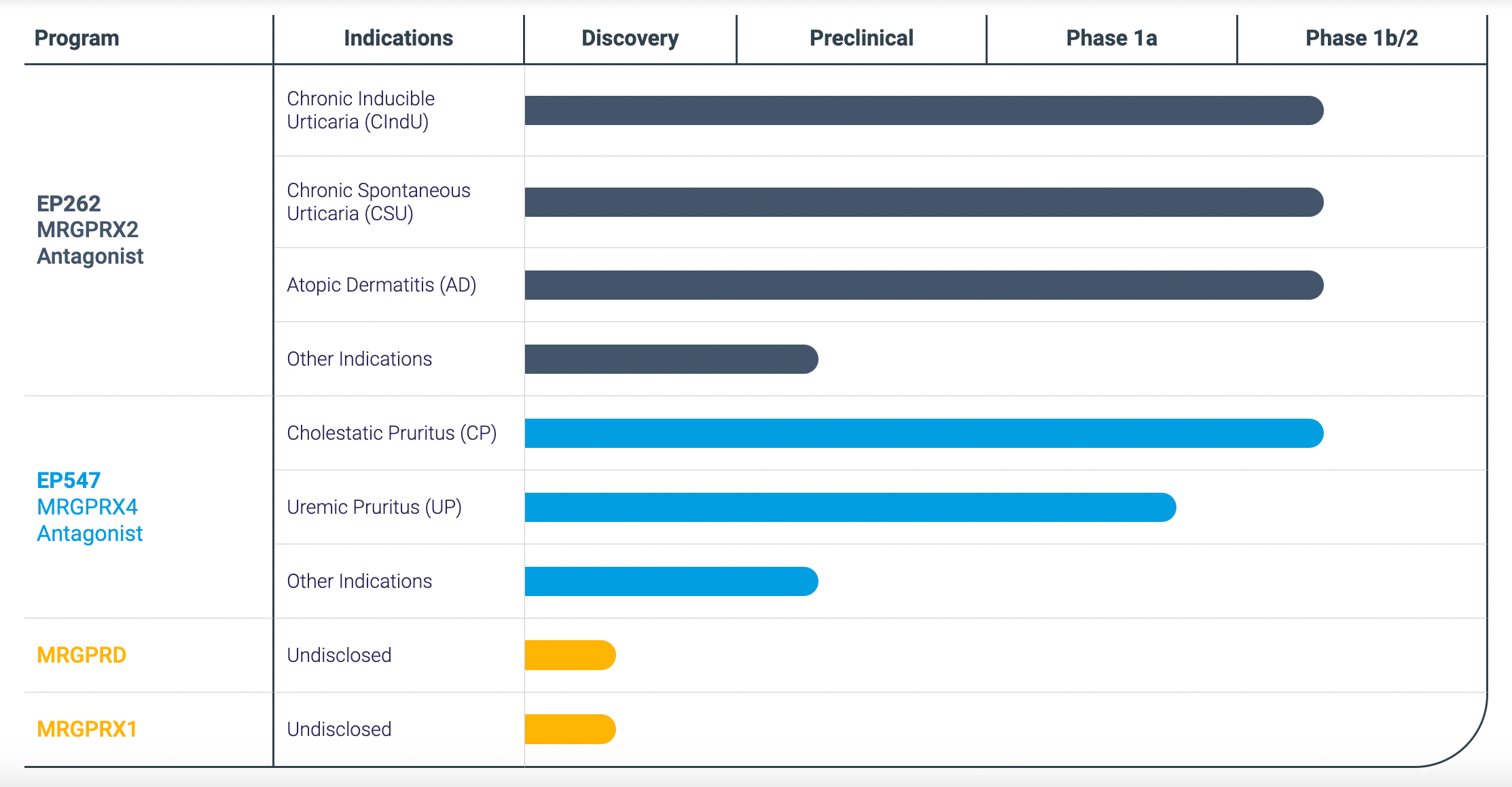
After the acquisition, Incyte plans the late-stage clinical research and marketing application for its two core products as shown in the chart below:
About EP262
In October 2023, Escient Pharmaceuticals (Escient), a biotechnology company based in California, announced the official launch of a clinical proof-of-concept study for their oral MRGPRX2 antagonist, EP262, aimed at treating chronic spontaneous urticaria. The company reported the administration of the first dose of EP262 to a study subject.
It is reported that Escient is developing EP262, a once-daily oral small molecule antagonist of the Mas-related G protein-coupled receptor X2 (MRGPRX2). EP262 has the potential to treat various mast cell-mediated diseases, with an initial focus on chronic urticaria and atopic dermatitis.
According to the announcement, Escient plans to evaluate the efficacy and safety of EP262 in a randomized, placebo-controlled study involving approximately 90 patients (NCT06077773).
CALM-CSU is a randomized, double-blind, placebo-controlled Phase 2 study designed to evaluate the efficacy, safety, tolerability, and pharmacokinetics of EP262 in approximately 90 patients with chronic spontaneous urticaria poorly controlled by antihistamines. Patients will be randomly assigned in a 1:1:1 ratio to receive either 50 mg of EP262, 150 mg of EP262, or a placebo, administered orally once daily for six weeks, followed by a washout period. The primary efficacy endpoint is the change from baseline in the 7-day Urticaria Activity Score (UAS7, a validated and widely used measure of disease activity).
At the end of November of the same year, Escient initiated a Phase IIa proof-of-concept study for EP262 in patients with atopic dermatitis (AD).
About MRGPRX2
MRGPRX2, a member of the Mas-related gene family, belongs to the GPCR family and has been found to be expressed in sensory neurons, mast cells, and keratinocytes. Additionally, MRGPRX2 mRNA is present in adipose tissue, esophagus, bladder, and lungs, with the highest levels found in the skin.
Research indicates that MRGPRX2 can be activated by basic secretagogues and neurokinins. Recently, several commonly used small molecule drugs (such as neuromuscular blocking agents, fluoroquinolones, and vancomycin) have been shown to activate these receptors under in vitro conditions, leading to mast cell degranulation, and thus causing IgE-mediated allergic reactions in allergic patients.
Upon activation of MRGPRX2, mast cells release histamine, tryptase, chymase, chemokines, and cytokines, which can result in urticaria, angioedema, type 2 inflammation (through the participation of the adaptive immune system), as well as chronic pruritus and pain.
The potential clinical treatment indications for targeting MRGPRX2 include chronic urticaria, neuroinflammation, atopic dermatitis, pruritus, and interstitial cystitis.
In September 2023, Evommune announced a strategic collaboration with Maruho to develop and commercialize the MRGPRX2 antagonist EVO756 in Japan. Evommune will receive up to $60 million in upfront and milestone payments, and will be eligible for tiered royalties on future sales of EVO756 in Japan.
About MRGPRX4
MRGPRX4 is a receptor specifically expressed in a subset of itch-sensing neurons within the dorsal root ganglia (DRG) connected to the skin. This receptor can be particularly activated by bile acids, bilirubin, and urobilinogen, substances that can accumulate in the skin and blood of patients suffering from cholestatic liver disease (CLD) and chronic kidney disease (CKD), causing severe itching. Unlike ordinary itching, this type of itch is often unrelievable by scratching and poorly responsive to existing antihistamines or other treatments, which are often accompanied by significant side effects and lack of specificity.
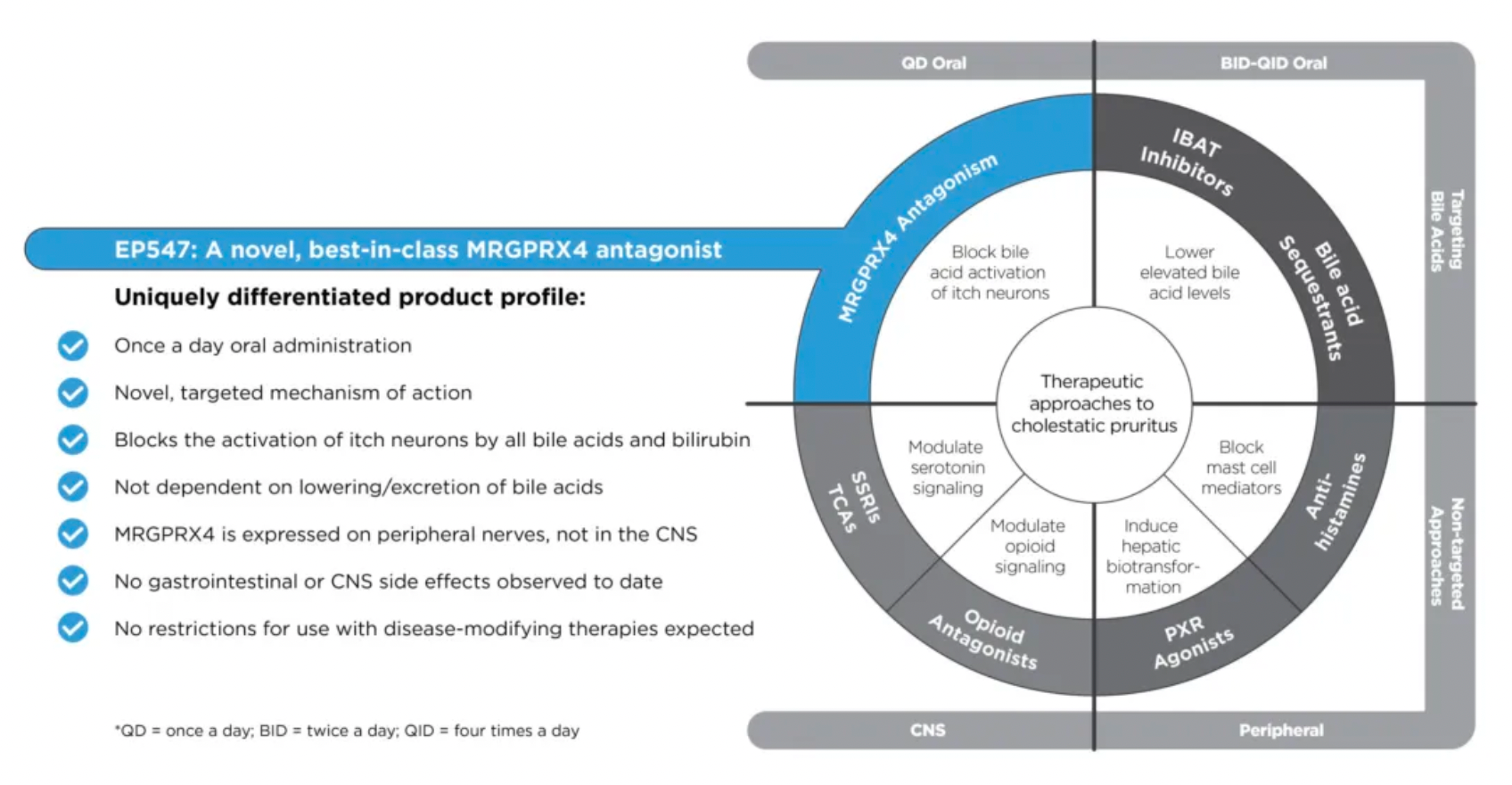
Escient is currently developing EP547, a small molecule antagonist targeting MRGPRX4, designed as a once-daily oral medication. Its high selectivity and potency allow EP547 to specifically target the MRGPRX4 receptor, potentially providing effective treatment for cholestatic and uremic itching without posing significant safety and tolerability issues. This therapy aims to address a significant unmet need in the current treatment landscape—finding a more precise and less side effect-prone medication to improve the quality of life for patients with these chronic conditions. By blocking the MRGPRX4 receptor, EP547 has the potential to directly intervene at the root cause of itching, bringing new hope to patients suffering from such refractory itching.
Currently, EP547 has entered Phase 2 clinical trials.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!