Genentech's Columvi Extends Survival in Relapsed/Refractory Large B-cell Lymphoma Trial
Genentech, part of the Roche Group, revealed today that their Phase III STARGLO trial of Columvi (glofitamab-gxbm) paired with gemcitabine and oxaliplatin demonstrated significant and clinically valuable outcomes compared to Rituxan (rituximab) combined with GemOx (R-GemOx). This study targeted individuals with relapsed or refractory diffuse large B-cell lymphoma who have undergone at least one previous therapy line and are unsuitable for autologous stem cell transplant, or have been treated with two or more prior therapy lines.
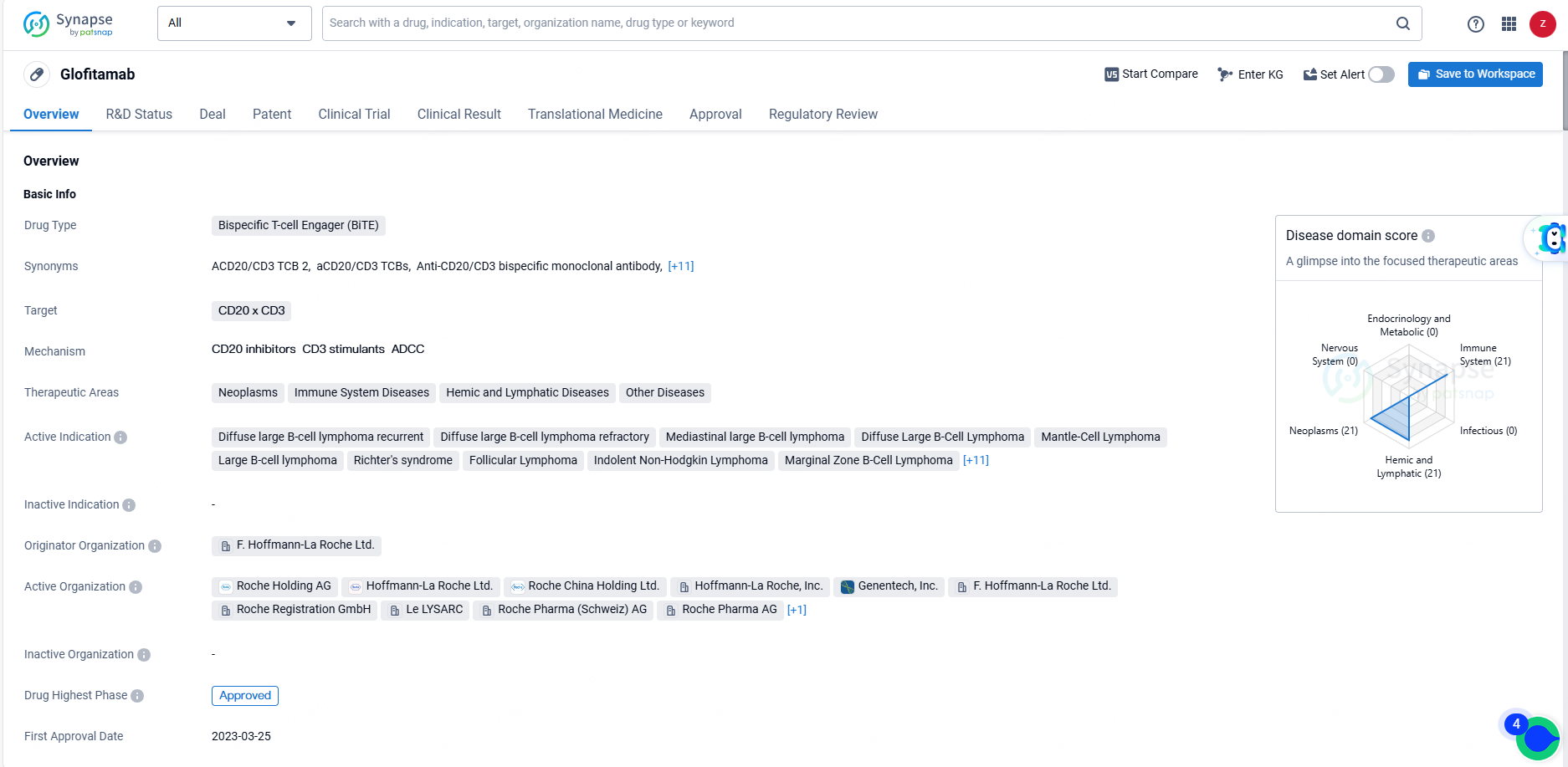
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Data were highlighted in the congress Press Briefing and were presented today during the Plenary Abstracts Session at the European Hematology Association 2024 Congress as a late-breaking oral presentation.
“The outcomes from the STARGLO study are the first to indicate that a CD20xCD3 bispecific antibody has the potential to impact the treatment of second or later-line DLBCL in patients who are ineligible for transplant and have limited treatment options,” stated Jeremy Abramson, M.D., director of the Jon and Jo Ann Hagler Center for Lymphoma at the Massachusetts General Hospital Cancer Center and principal investigator of the STARGLO study.
The pre-specified exploratory subgroup analyses yielded similar results, exhibiting consistency among the clinically significant stratification factors of therapy line and the outcome of the most recent therapy. However, regional variations were noted, though the interpretation is constrained by the exploratory nature of these analyses and the small subgroups with broad confidence intervals.
“This represents an initial step in advancing Columvi combinations in earlier lines of treatment to address the urgent needs of 40% of patients who will experience relapse or have refractory disease with limited options available,” commented Levi Garraway, M.D., Ph.D., Genentech’s chief medical officer and head of Global Product Development. “Additionally, patients don’t have to delay initiating Columvi treatment. This is particularly crucial for those with highly aggressive diseases that pose a risk of rapid disease progression.”
Columvi is the first CD20xCD3 bispecific antibody to display a survival advantage in DLBCL in a randomized Phase III trial, showcasing the potential of this therapeutic combination to enhance survival outcomes in earlier treatment phases. Historically, the standard second-line therapy for R/R DLBCL patients has involved high-dose chemotherapy followed by stem-cell transplant; however, not all patients with R/R DLBCL are suitable candidates owing to age or other medical conditions.
Although newer therapies are emerging, significant barriers still exist for many, underscoring the need for alternative treatment options. Columvi, administered as a fixed-duration treatment, offers patients with R/R DLBCL the possibility of a definitive treatment end and a treatment-free period, unlike continuous therapies.
Columvi is also under investigation for other aggressive, hard-to-treat lymphomas and was recently granted Breakthrough Therapy Designation by the FDA for treating adult patients with relapsed or refractory mantle cell lymphoma who have undergone at least two prior therapies, based on findings from the Phase I/II NP30179 study.
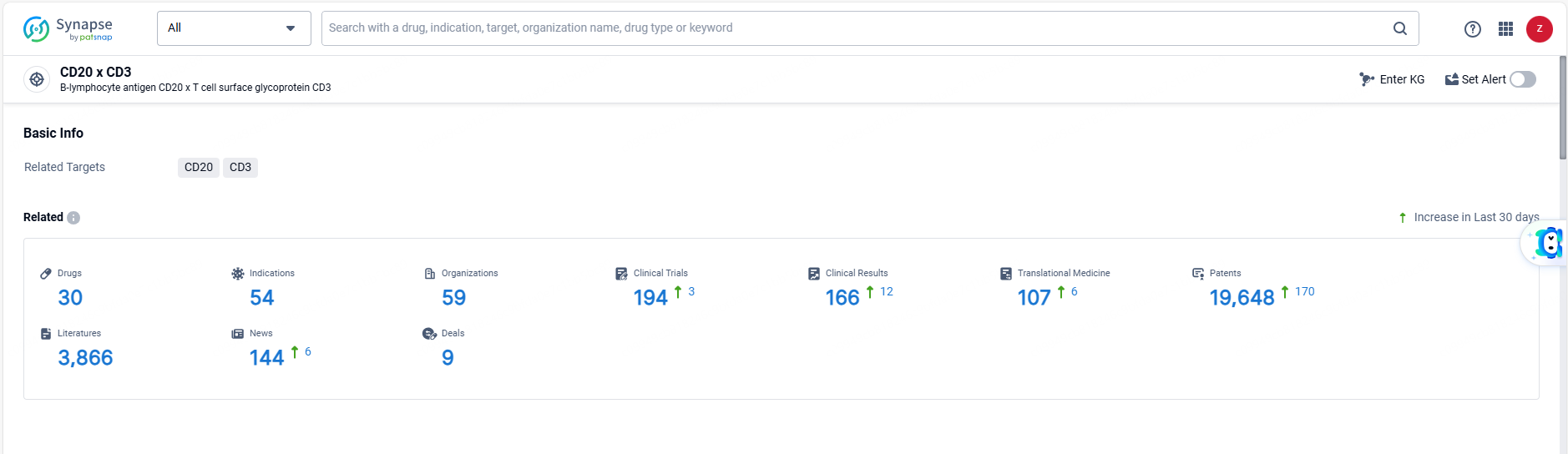
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 18, 2024, there are 30 investigational drugs for the CD20 and CD3 target, including 54 indications, 59 R&D institutions involved, with related clinical trials reaching 194, and as many as 19648 patents.
Glofitamab's approval and regulatory status position it as a significant advancement in the treatment of lymphomas and related conditions, offering hope for patients who have not responded to conventional therapies. Its unique mechanism of action and broad therapeutic potential make it a valuable addition to the pharmaceutical landscape in the field of biome.