Eluminex Biosciences Initiates Phase 1b LOTUS Study for EB-105, a Trispecific Fusion Antibody Treating DME
Eluminex Biosciences, a clinical stage, privately held biotechnology company focused on the development of advanced protein therapeutics for vision-threatening diseases announced the first dosing of EB-105 in patients with DME.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 The LOTUS trial, serving as a pioneering human study, is an open-label, multicenter investigation involving a single injection and dose-escalation of EB-105, Eluminex’s leading ophthalmology asset, conducted across four locations in the United States. EB-105 has demonstrated robust inhibition of VEGF-A/VEGFR-2, Ang-2/Tie-2, and IL-6/IL-6R signaling pathways in preclinical trials.
The LOTUS trial, serving as a pioneering human study, is an open-label, multicenter investigation involving a single injection and dose-escalation of EB-105, Eluminex’s leading ophthalmology asset, conducted across four locations in the United States. EB-105 has demonstrated robust inhibition of VEGF-A/VEGFR-2, Ang-2/Tie-2, and IL-6/IL-6R signaling pathways in preclinical trials.
Elevated vitreous levels of IL-6, an inflammatory cytokine associated with endothelial cell barrier dysfunction leading to vascular leakage, are observed in patients suffering from retinal conditions such as diabetic macular edema, diabetic retinopathy, neovascular age-related macular degeneration, and retinal vein occlusion.
Dr. Ashkan Abbey, MD, Director of Clinical Research at Texas Retina Associates, became the first retinal surgeon globally to administer EB-105 in a clinical setting. “Retina specialists are eager to incorporate IL-6 pathway inhibition into their practice. There is a need for novel agents to manage these challenging patient cases. We hope that EB-105 will result in improvements in visual acuity, duration of response, and retinal drying,” he said.
“EB-105 signifies a major advancement in the emerging field of polyvalent protein engineering,” noted Charles Semba, MD, Eluminex’s Chief Medical Officer. “For patients, it holds the potential to provide more comprehensive coverage of known biological pathways involved in diabetic retinopathy, and may lead to better outcomes than current therapies,” he added.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
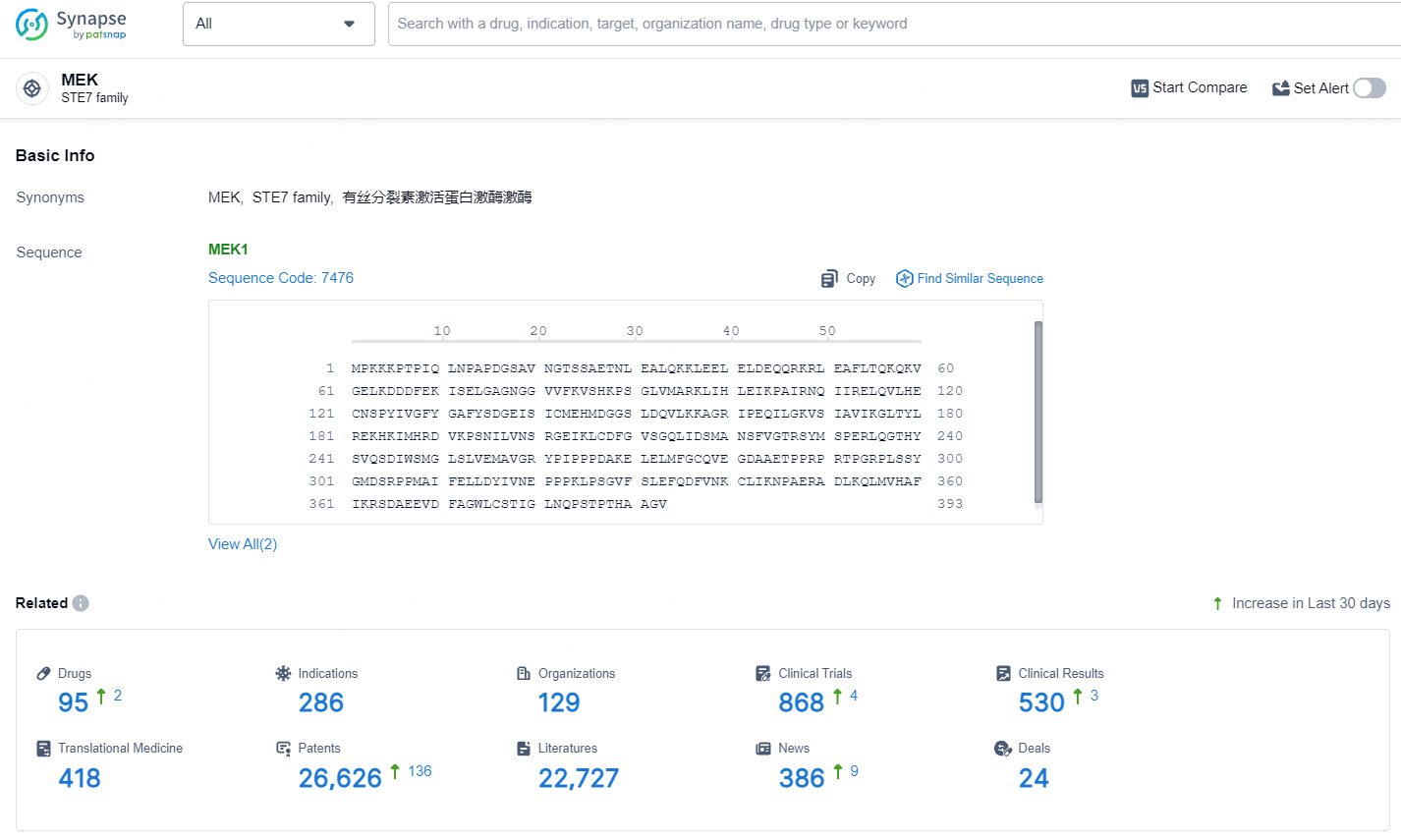
According to the data provided by the Synapse Database, As of July 18, 2024, there are 95 investigational drugs for the MEK target, including 286 indications, 129 R&D institutions involved, with related clinical trials reaching 868, and as many as 26626 patents.
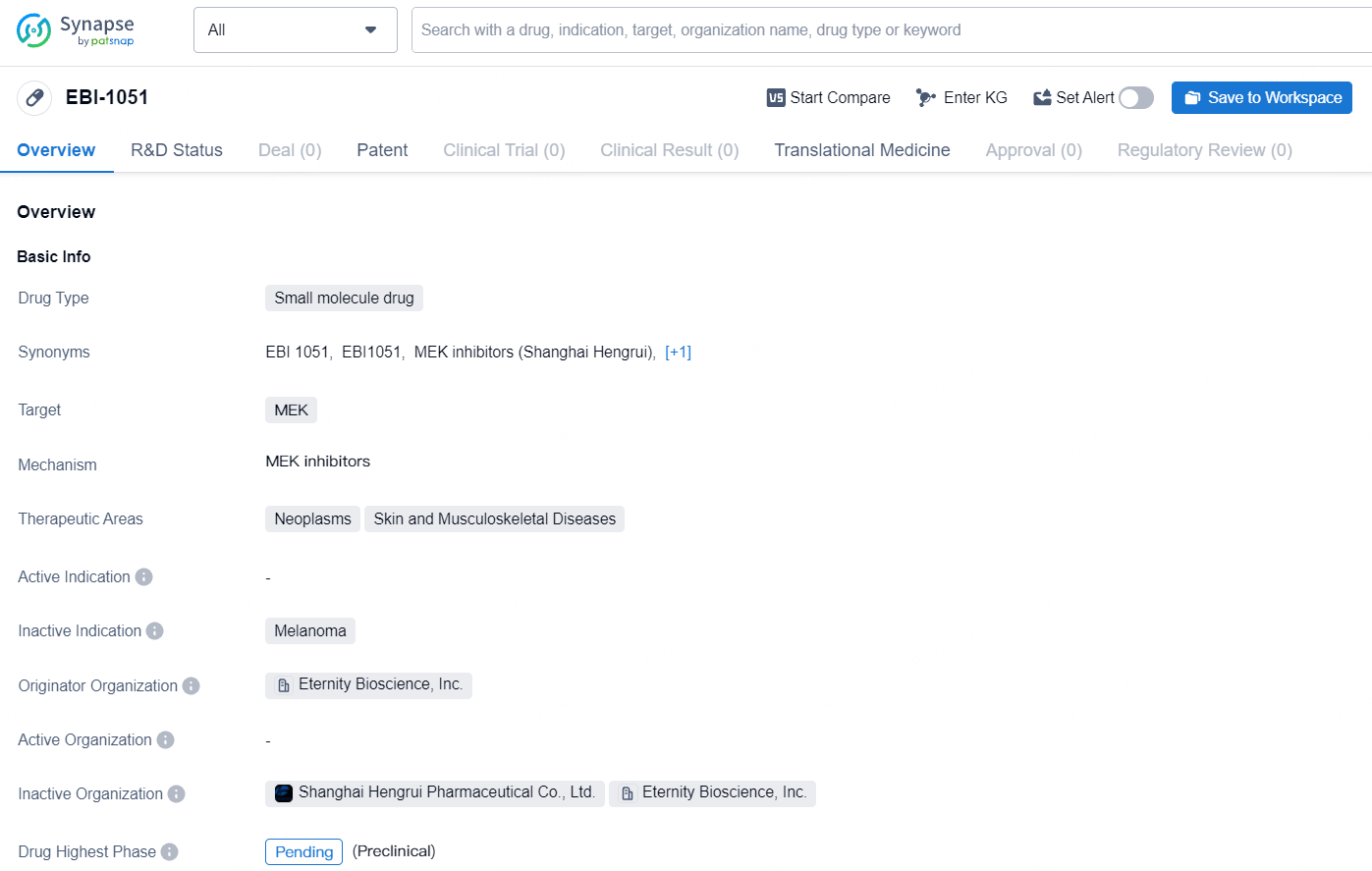
EBI-1051 is a small molecule drug with a focus on targeting MEK for the treatment of neoplasms, skin, and musculoskeletal diseases. With Eternity Bioscience, Inc. leading its development, the drug is still in the pending phase of development, indicating ongoing clinical trials and further research.