Is Tadalafil/finasteride approved by the FDA?
Yes, tadalafil and finasteride (Entadfi) is FDA approved. The combination of these two drugs was approved by the FDA to treat signs and symptoms of BPH, providing relief for men with an enlarged prostate.
Uses
Tadalafil and finasteride are used together to alleviate the symptoms of BPH, a condition characterized by an enlarged prostate gland. This combination helps to improve urine flow and reduce symptoms such as difficulty in starting urination and the need to urinate frequently or urgently.
Dosage
The usual adult dosage for treating BPH with tadalafil and finasteride is one capsule (containing 5 mg of tadalafil and 5 mg of finasteride) taken orally once daily. The capsule should be taken without food at approximately the same time each day, and the treatment duration is up to 26 weeks.
Administration Instructions
- Follow Prescription: Take the medication exactly as prescribed by your doctor. Do not take more than one dose per day.
- Missed Dose: If you miss a dose, take it as soon as possible, but do not take more than one dose per day.
- Storage: Store the medication at room temperature away from moisture and heat.
Warnings and Precautions
- Nitrate Medications: Do not take tadalafil and finasteride with nitrate medications (e.g., nitroglycerin) or recreational drugs containing nitrates (e.g., "poppers"), as this can cause a sudden and serious decrease in blood pressure.
- Pregnancy: This medication is not for use in women and can cause birth defects if a woman is exposed to it during pregnancy. Pregnant women should not handle crushed or open capsules.
- Allergies: Inform your doctor if you have any allergies to tadalafil, finasteride, or any other medications.
- Pre-existing Conditions: Disclose any history of heart problems, pulmonary hypertension, liver or kidney disease, vision or hearing issues, bleeding problems, or any deformities of the penis to your doctor.
Side Effects
Common side effects of tadalafil and finasteride may include:
- Headache
- Indigestion
- Back pain
- Muscle aches
- Loss of interest in sex, impotence
- Enlarged or painful breasts
- Stuffy or runny nose
- Flushing (sudden warmth, redness, or tingly feeling)
Serious side effects requiring immediate medical attention include:
- Allergic reactions (hives, difficult breathing, swelling in the face or throat)
- Severe skin reactions (fever, sore throat, burning eyes, skin pain, red or purple skin rash with blistering and peeling)
- Heart attack symptoms (chest pain or pressure, pain spreading to the jaw or shoulder, nausea, sweating)
- Painful or prolonged erection lasting longer than 4 hours
- Breast lumps or pain, nipple discharge
- Ringing in the ears or sudden hearing loss
- Vision changes or sudden vision loss
Drug Interactions
Tadalafil and finasteride can interact with various medications. It is crucial to inform your doctor about all your current medications, including prescription and over-the-counter drugs, vitamins, and herbal products. Notably, avoid taking this medication with:
- Medicines for pulmonary arterial hypertension (e.g., riociguat)
- Other drugs for erectile dysfunction or BPH
- Certain antibiotics, antifungal medications, and HIV/AIDS treatments
Conclusion
Tadalafil and finasteride (Entadfi) is an FDA-approved medication for the treatment of benign prostatic hyperplasia (BPH) in men. It effectively relieves symptoms associated with an enlarged prostate. However, it should be used with caution, considering potential side effects and interactions with other medications. Always consult your healthcare provider for personalized advice and treatment plans.
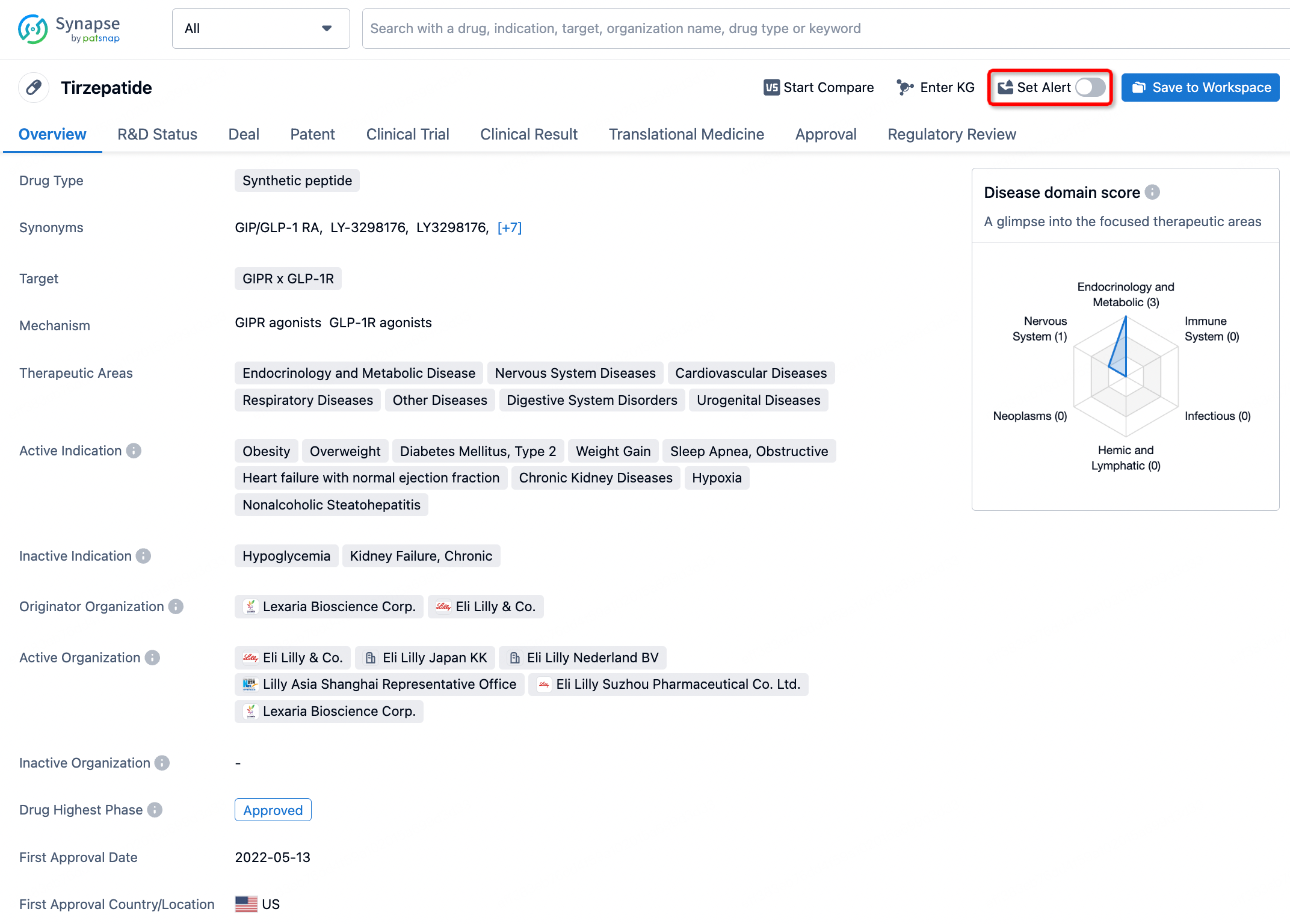
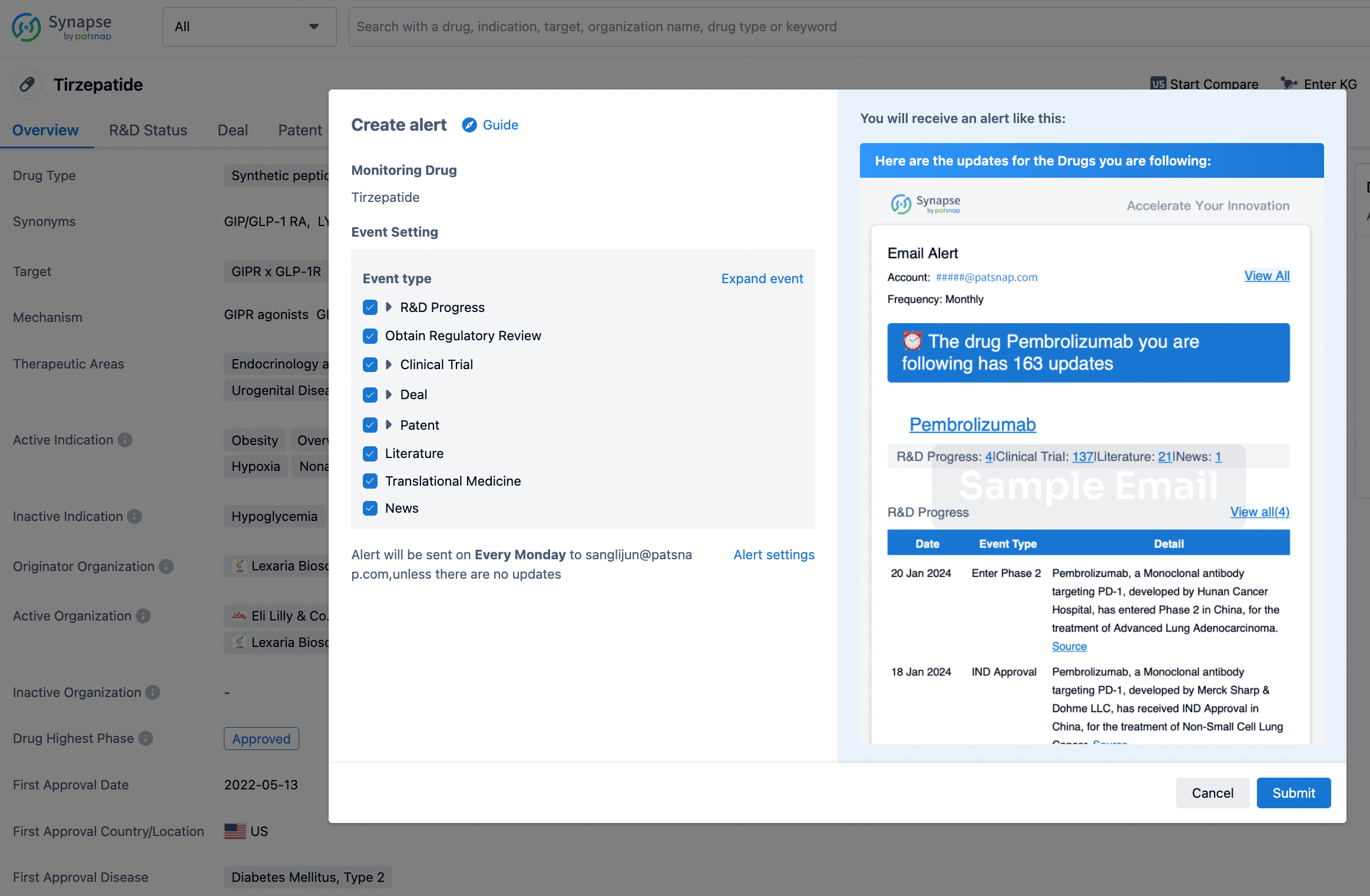
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!