EMA Approves Review of Tisotumab Vedotin for Advanced Cervical Cancer Therapy
Genmab A/S in collaboration with Pfizer, Inc. has shared the news that the European Medicines Agency has commenced evaluation of the submitted documents for the marketing authorization of tisotumab vedotin. This medicinal product is an antibody-drug conjugate, specifically created to address the needs of adults who are suffering from cervical cancer that has either returned or spread, and who have experienced disease advancement despite undergoing systemic treatment. Upon receiving the green light, tisotumab vedotin is poised to become the inaugural ADC to acquire marketing approval within the European Union for the treatment of individuals affected by cervical cancer.
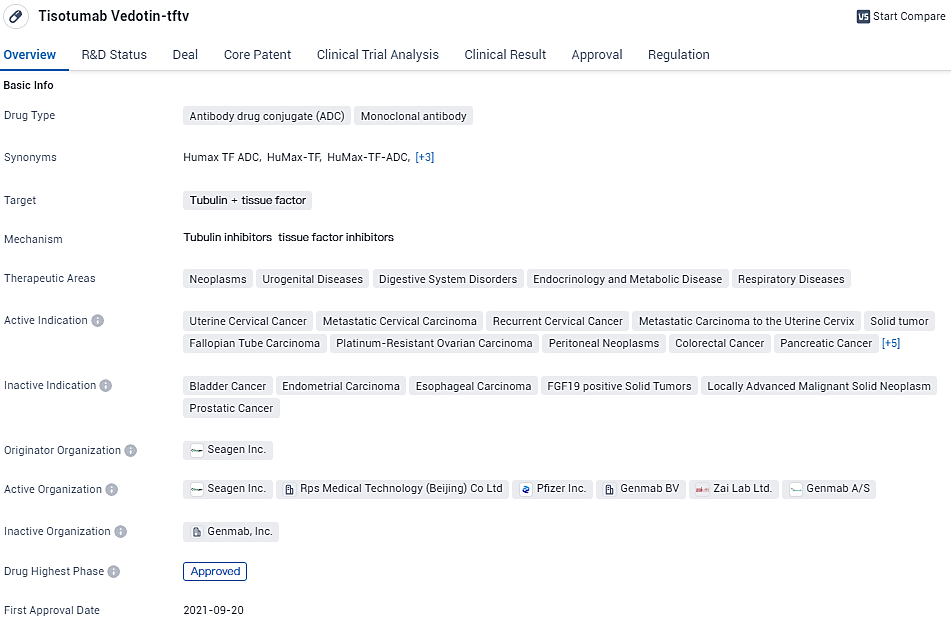
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The MAA submission is anchored in outcomes from the international, multicenter, Phase 3 innovaTV 301 trial. This pivotal study showcased the enhanced efficacy of tisotumab vedotin in extending overall survival, delaying disease progression, and achieving a verified response in treating individuals with recurrent or metastatic cervical cancer after prior therapy, outperforming standard chemotherapy regimens.
Included in the MAA dossier were findings from the innovaTV 204 trial—a key Phase 2 research focusing on the use of TIVDAK as a single-agent treatment for similar patient groups. Tisotumab vedotin's safety parameters reported in innovaTV 301 align with the profile documented within the prescribing guidelines in the United States.
"With the MAA assessment process underway, we've reached a crucial step in our mission to offer a novel treatment for those battling with recurring or advanced stages of cervical cancer," remarked Jan van de Winkel, Ph.D., Genmab's CEO. "Given the urgent requirement for new treatments for these individuals, we are unwavering in our pursuit to present better options for women suffering from this aggressive illness."
Highlighting the advancement, Roger Dansey, M.D., Pfizer's Chief Development Officer for Oncology, noted, "This milestone underscores our efforts in widening access to tisotumab vedotin for patients enduring recurrent or metastatic cervical cancer. As we push forward, we are committed to working in tandem with health regulators, striving to introduce an innovative treatment option for those contending with this challenging condition."
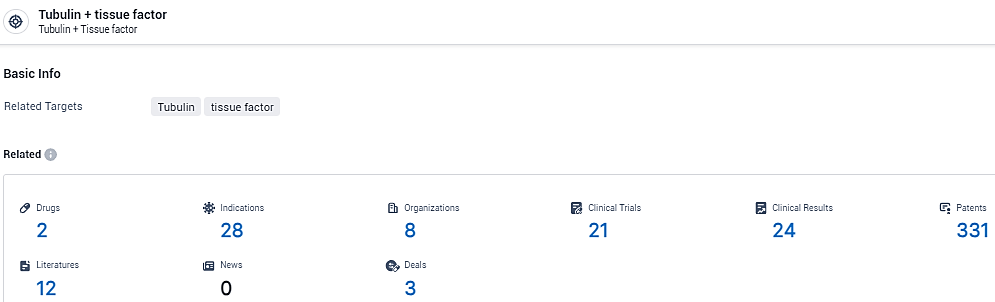
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 2 investigational drugs for the Tubulin and tissue factor target, including 28 indications, 8 R&D institutions involved, with related clinical trials reaching 21, and as many as 331 patents.
tisotumab vedotin-tftv is an approved ADC and monoclonal antibody that targets tubulin and tissue factor. It has shown promise in the treatment of various cancers and has received regulatory approvals, including priority review and accelerated approval. The drug has reached the highest phase of development globally and is currently in phase 3 in China. Its first approval was granted in the United States, and it is indicated for multiple cancer types, including uterine cervical cancer, colorectal cancer, and non-small cell lung cancer.