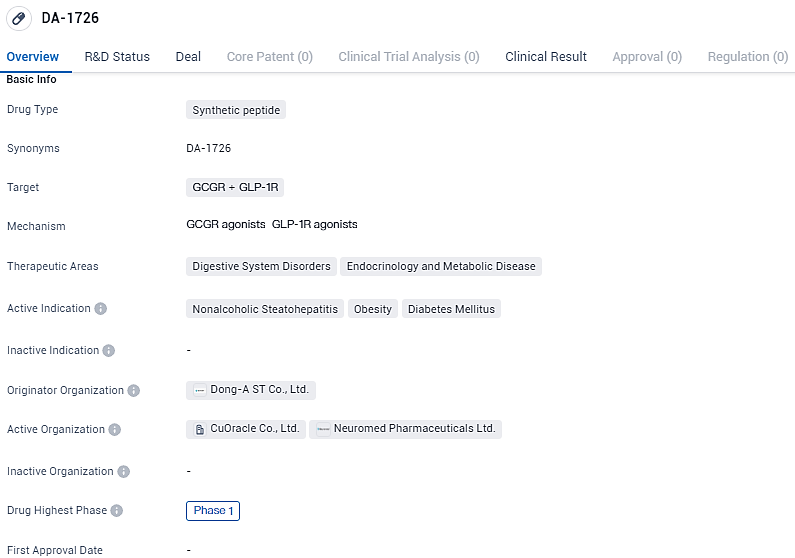
FDA Approves NeuroBo Pharmaceuticals' Phase 1 Study of Obesity Drug DA-1726
NeuroBo Pharmaceuticals, Inc. has received approval from the U.S. Food and Drug Administration for its new drug application for DA-1726, an innovative oxyntomodulin derivative that acts as an agonist for both glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor (GCGR). The pharmaceutical firm intends to commence a Phase 1 study targeting obesity management, scheduled to begin within the initial six months of the current year.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The authorization of the Investigational New Drug application for DA-1726 enables us to initiate our Phase 1 trials, targeting a combined GLP-1 and glucagon receptor with the potential to revolutionize obesity treatment," declared Hyung Heon Kim, the CEO of NeuroBo Pharmaceuticals.
Kim further remarked, "Our anticipation is that DA-1726 could exhibit an enhanced safety profile compared to existing GLP-1 agonist treatments, given its unique mechanism that simultaneously engages GLP1R and glucagon receptors. We are excited about the prospect of administering DA-1726 to the inaugural participant before mid-year and are aiming for preliminary trial outcomes by mid-2025."
The Phase 1 clinical research is set to be an organized, placebo-influenced, double-masked, parallel-group sequential investigation dedicated to evaluating the safety profile, dose tolerance, pharmacokinetic behavior, and pharmacodynamic effects of DA-1726 administered in single and staggered increased doses to overweight but otherwise healthy individuals.
The preliminary stage of the trial will focus on single dose escalation, with an anticipated enrollment of roughly 45 volunteers who will be divided into 5 distinct cohorts. Within each group, participants will be allocated in a 6:3 ratio to receive either DA-1726 or a placebo. The succeeding stage will concentrate on incremental multiple dosages, aiming to register about 36 individuals split into 4 cohorts, with each cohort slated to receive DA-1726 or a placebo over four weeks.
The central goal of the study is to monitor the safety and ease of tolerance to DA-1726 by recording any adverse effects, significant medical incidents, adverse reactions appearing post-treatment, and any adverse events necessitating a cessation of the treatment. Additional objectives involve examining the pharmacokinetics of DA-1726, which will be done through serum concentration measurements over time and scrutinizing metabolites at the highest administered doses. Investigative objectives will survey the influence of DA-1726 on an array of health metrics, such as metabolic and cardiac functions, cholesterol profiles, total body weight, abdominal girth, and overall body mass index, among others.
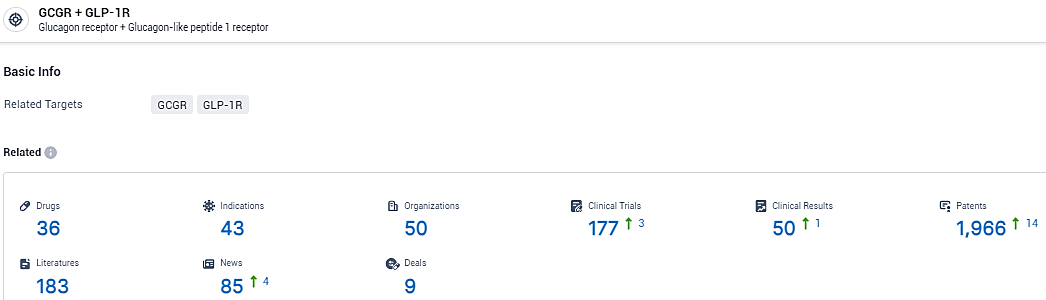
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 36 investigational drugs for the GCGR and GLP-1R target, including 43 indications, 50 R&D institutions involved, with related clinical trials reaching 177, and as many as 1966 patents.
DA-1726 targets the GCGR and GLP-1R and is indicated for the treatment of Nonalcoholic Steatohepatitis, Obesity, and Diabetes Mellitus. With its potential to address significant unmet medical needs in these therapeutic areas, DA-1726's progression through clinical development will be closely monitored by the pharmaceutical industry and healthcare professionals.