Ascendis Pharma Launches YORVIPATH® for Adult Hypoparathyroidism in Germany and Austria
Ascendis Pharma A/S recently declared the commercial availability of YORVIPATH® (palopegteriparatide, developed as TransCon PTH) in Germany and Austria. This medication serves as a parathyroid hormone substitute therapy and is designed to address chronic hypoparathyroidism in adult patients. This release marks the second introduction of a product formulated using the TransCon technology platform by Ascendis Pharma.
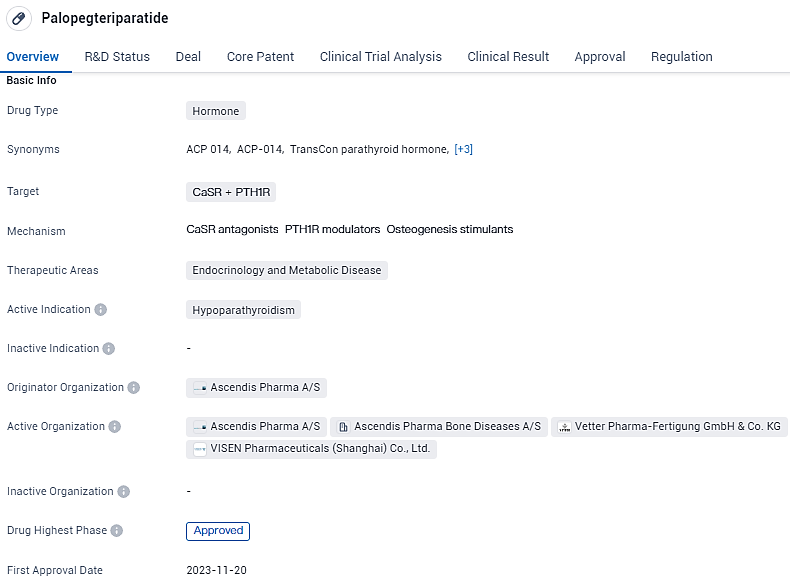
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"It is with enthusiasm that we announce the introduction of YORVIPATH, a novel parathyroid hormone (PTH)-based therapy, which aims to fulfill the specific treatment requirements highlighted by the hypoparathyroidism patient community," proclaimed Jan Mikkelsen, the President and CEO of Ascendis Pharma.
Leveraging our extensive and growing commercial network, we are strategically poised to enhance the accessibility of YORVIPATH along with our other authorized products. This expansion is aimed at catering to the global demand from healthcare professionals and their patients," further stated Jan Mikkelsen.
YORVIPATH (palopegteriparatide, also known as TransCon PTH in its development phase), is a once-daily injectable prodrug that consistently regulates parathyroid hormone levels, ensuring they remain within the normal range throughout the 24-hour dosing cycle. The European Commission conferred marketing authorization to YORVIPATH in November 2023, approving it as a PTH replacement therapy for adult patients suffering from chronic hypoparathyroidism.
An endorsement for YORVIPATH by the United Kingdom's Medicines & Healthcare Products Regulatory Agency is anticipated within the initial segment of 2024. Meanwhile, the U.S. Food & Drug Administration has scheduled a Prescription Drug User Fee Act (PDUFA) goal date of May 14, 2024, to finalize its evaluation of the New Drug Application submitted by Ascendis Pharma for the use of TransCon PTH in adult patients with chronic hypoparathyroidism.”
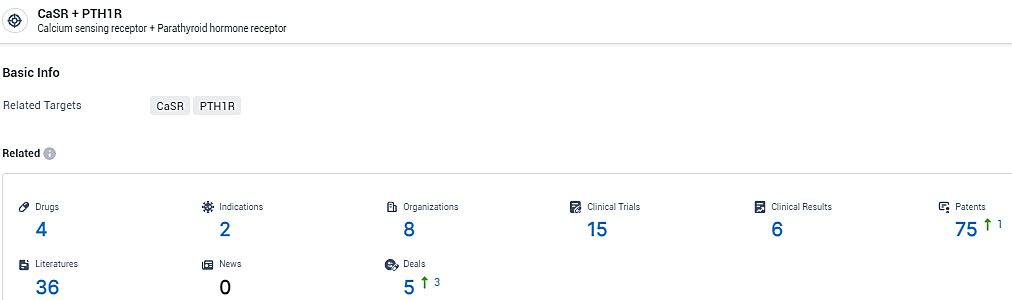
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 8, 2024, there are 4 investigational drugs for the CaSR and PTH1R target, including 2 indications, 8 R&D institutions involved, with related clinical trials reaching 15, and as many as 75 patents.
Palopegteriparatide has been granted priority review status, which suggests that it addresses an unmet medical need or offers significant benefits over existing treatments. Additionally, it has been designated as an orphan drug, indicating that it is intended to treat a rare disease or condition.