Alvotech Reveals Promising Preliminary Results for Potential Prolia® and Xgeva® Equivalent, AVT03
The international biotechnology corporation Alvotech, known for its expertise in creating and producing biosimilar drugs for a global patient base, reported encouraging preliminary outcomes from a pharmacokinetic analysis regarding AVT03. This particular biosimilar contender is developed as an alternative to the denosumab-containing medications Prolia® and Xgeva®.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The investigation into the dynamics and safety profile of AVT03, in comparison to Prolia®, among healthy adults has reached its primary goals. Ongoing projects include a pivotal study to verify AVT03's effectiveness in patients and a pharmacokinetic (PK) analysis that juxtaposes AVT03 with Xgeva® in a healthy adult cohort.
"Our AVT03 initiative is progressing impressively," shared Joseph McClellan, the Lead Scientist at Alvotech. "Achieving this key point, along with the promising preliminary outcomes from other trials, solidly affirms Alvotech's expertise and the robustness of our biosimilar development framework."
Prolia, or denosumab, is recommended for treating osteoporosis in women who have experienced menopause and for tackling bone density reduction in adults who are at an elevated risk of breaks. Xgeva, also a form of denosumab but with varied application, is aimed at preventing bone-related complications like abnormal fractures in individuals with severe bone-affecting cancers and is also utilized in addressing giant cell tumor in the bone.
In the fiscal period ending September 30, 2023, the accumulated net income from global Prolia and Xgeva sales surpassed the US$6 billion mark, as per the quarterly financial disclosures of the manufacturing company.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights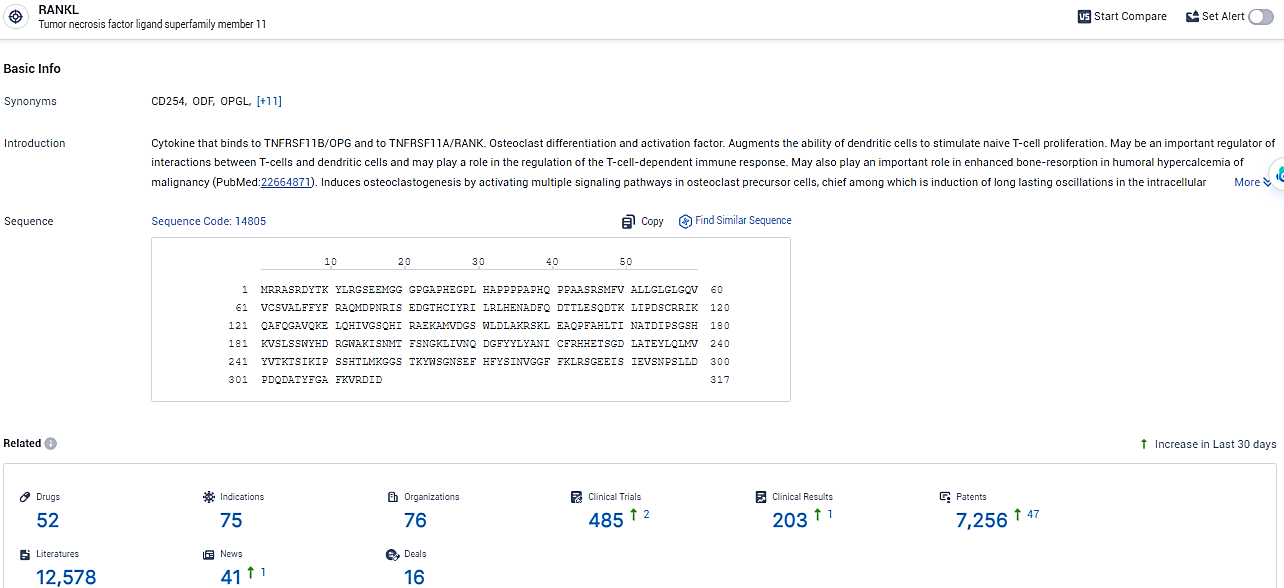
According to the data provided by the Synapse Database, As of February 5, 2024, there are 52 investigational drugs for the RANKL target, including 75 indications, 76 R&D institutions involved, with related clinical trials reaching 485, and as many as 7256 patents.
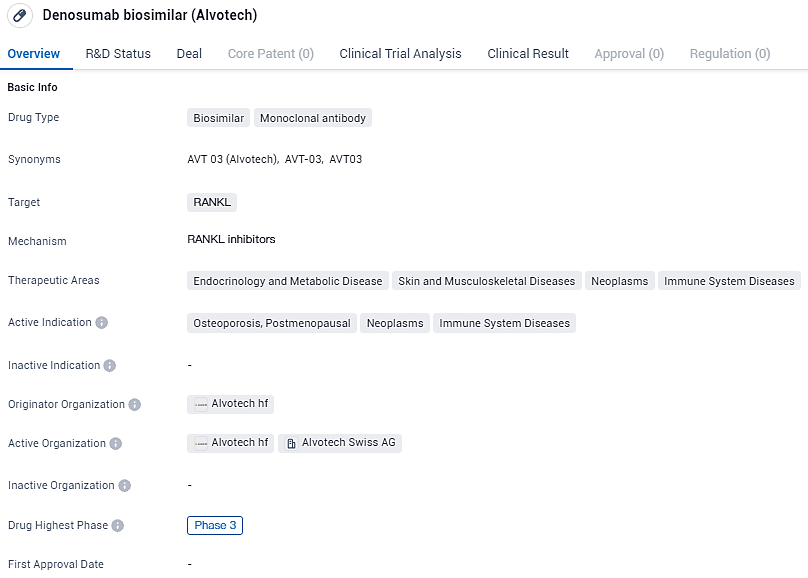
AVT03 is a monoclonal antibody drug that targets RANK and has potential therapeutic applications in various areas, including endocrinology and metabolic diseases, skin and musculoskeletal diseases, neoplasms, and immune system diseases. Its active indications include osteoporosis in postmenopausal women, neoplasms, and immune system diseases.