EMA Validates Bristol Myers Squibb's Subcutaneous Nivolumab Application
Bristol Myers Squibb revealed that the European Medicines Agency has accepted the extension application for a novel administration route of Opdivo (nivolumab). This extension comprises a new pharmaceutical form and a new dosage strength for various previously approved adult solid tumor indications. These indications include use as a monotherapy, monotherapy maintenance after the completion of nivolumab and ipilimumab combination therapy, or in combination with chemotherapy or cabozantinib. The extension is supported by data from the Phase 3 CheckMate -67T study.
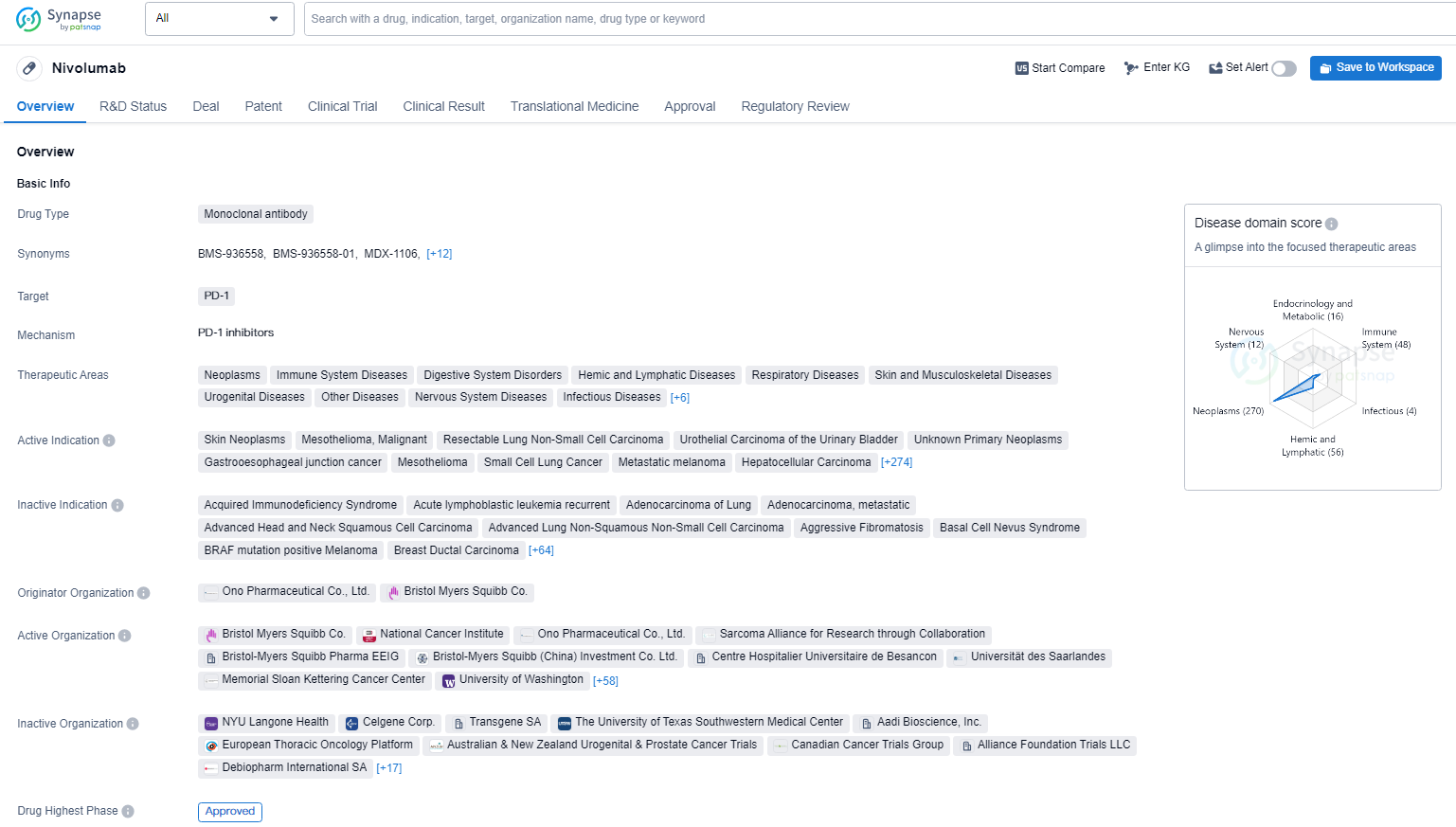
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Subcutaneous (SC) nivolumab offers an innovative approach to administering Opdivo to cancer patients, potentially transforming the treatment process and significantly shortening the administration time to just three-to-five minutes through a single injection. This method provides equivalent care quality to that of intravenous (IV) Opdivo but drastically reduces the time patients spend at infusion centers, allowing them to prioritize other aspects of their lives,” stated Susan Parker, Vice President, Global Program Lead, Product Design & Development at Bristol Myers Squibb.
“Our mission is to enhance the patient experience by developing advanced medicines, and we're actively exploring novel formulations across our extensive portfolio. Our goal is to collaborate with the EMA to advance the application of this subcutaneous Opdivo option,” added Parker.
In the Phase 3 CheckMate -67T trial, subcutaneous nivolumab proved to be noninferior in terms of Cavgd28 and Cminss, the primary endpoints, compared to IV Opdivo in patients with advanced or metastatic clear cell renal cell carcinoma who had undergone no more than two prior lines of systemic therapy.
Moreover, subcutaneous nivolumab achieved noninferiority in the key secondary endpoint of objective response rate, as evaluated by Blinded Independent Central Review, against its IV counterpart. The safety profile of SC nivolumab was consistent with that of the IV version. The results regarding pharmacokinetics, efficacy, and safety from CheckMate -67T were unveiled at the 2024 American Society of Clinical Oncology Genitourinary Cancers Symposium, along with additional safety analyses and patient-reported outcomes."
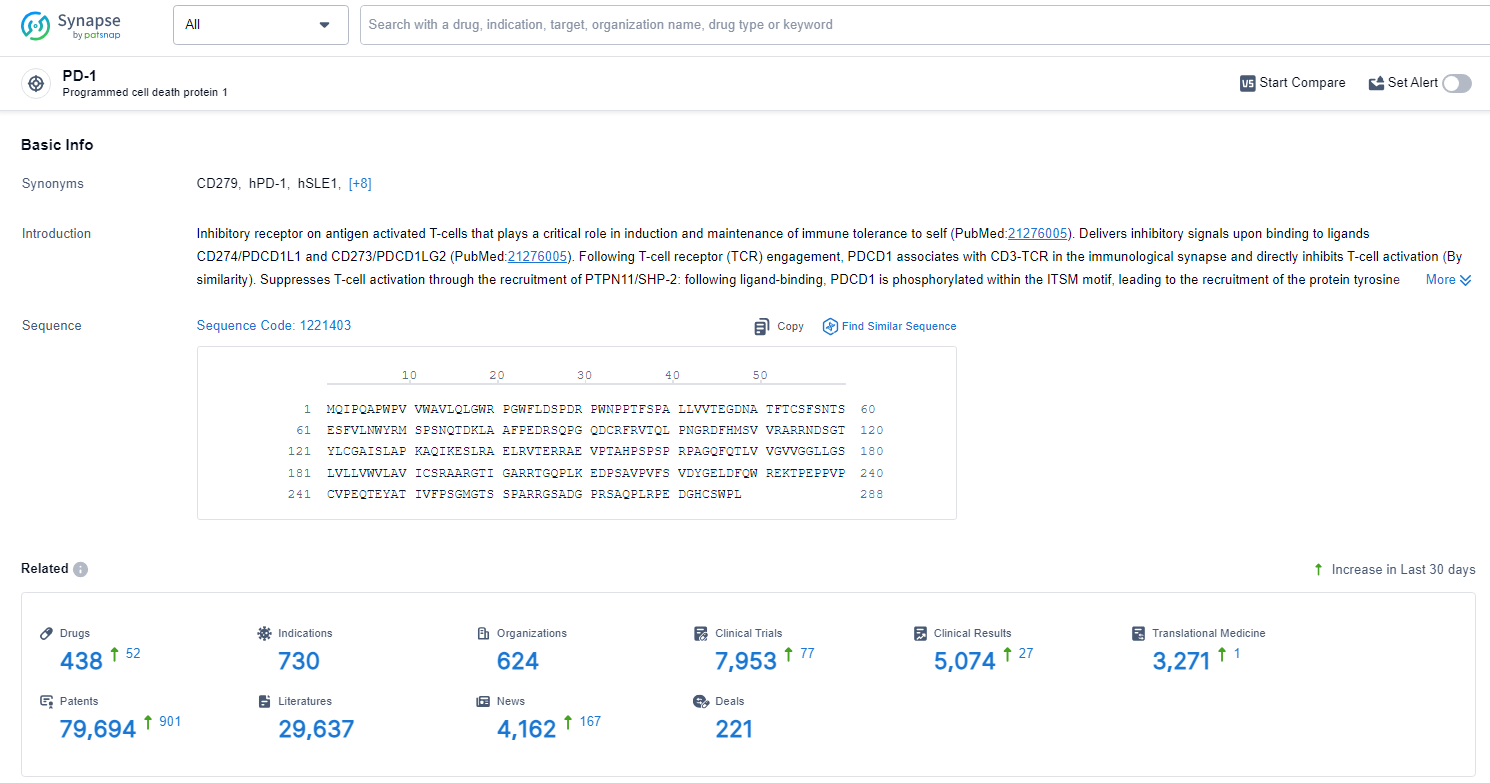
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 438 investigational drugs for the PD-1 target, including 730 indications, 624 R&D institutions involved, with related clinical trials reaching 7953, and as many as 79694 patents.
Nivolumab has shown promising results in the treatment of a wide range of cancers and has been approved for use in multiple countries. Its regulatory designations indicate that it has the potential to address unmet medical needs and provide significant benefits to patients. As a monoclonal antibody targeting PD-1, Nivolumab represents a significant advancement in the field of biomedicine and has the potential to improve outcomes for patients with various types of cancer.