Encouraging Phase 2 Results of NBI-1117568 in Adult Schizophrenia Show Potential Efficacy
Neurocrine Biosciences, Inc. (Nasdaq: NBIX) has unveiled encouraging preliminary findings from its Phase 2 clinical evaluation of NBI-1117568 (NBI-'568) among schizophrenic adult patients. NBI-'568 represents the pioneering, orally administered, muscarinic M4 receptor-selective agonist being pursued for schizophrenia therapy.
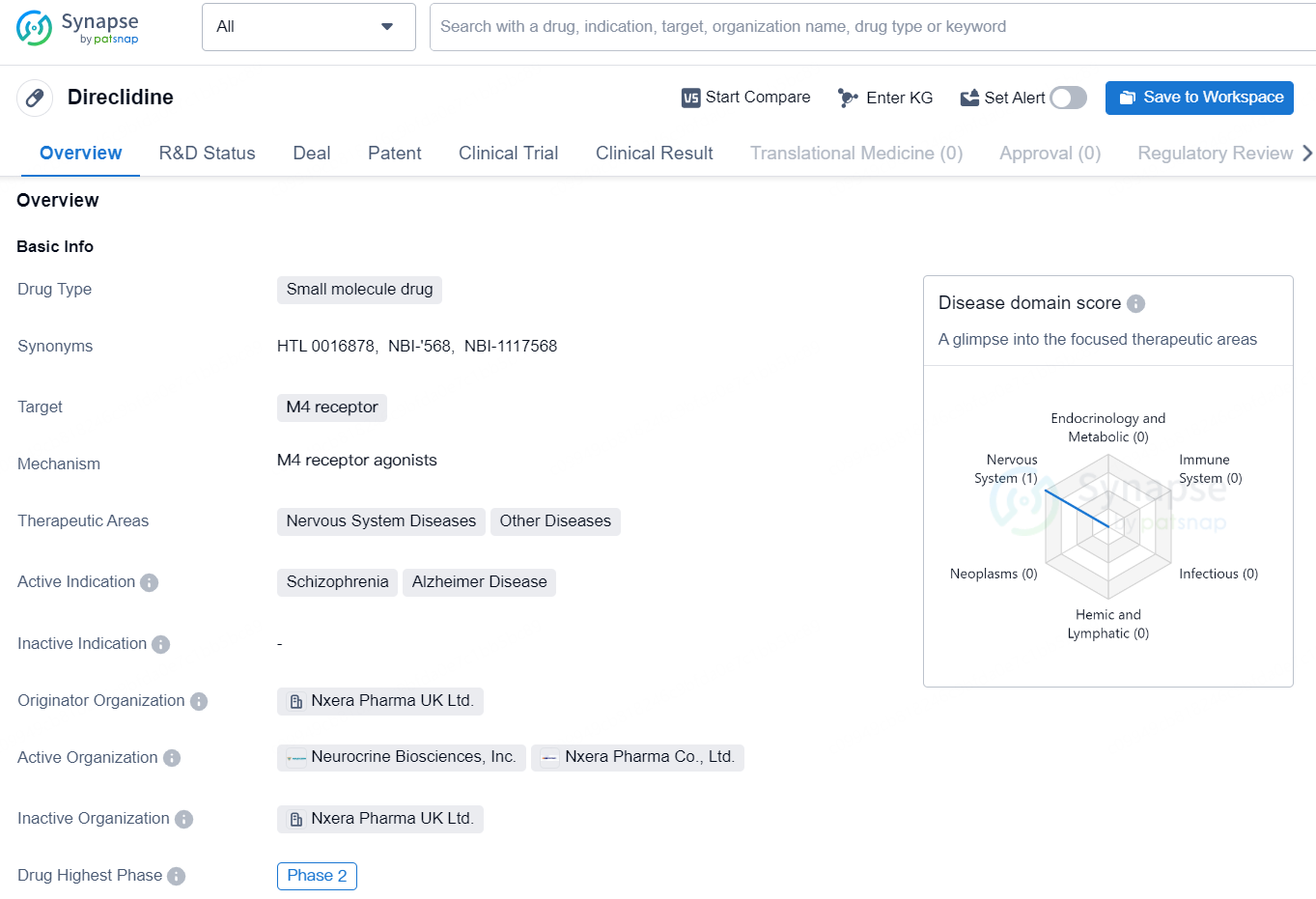
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The NBI-'568-SCZ2028 dose optimization study achieved its primary objective for the 20 mg daily dosage, exhibiting a clinically relevant and statistically substantial decline in PANSS total score from baseline at Week 6, with a 7.5-point mean reduction compared to placebo (p=0.011, effect size 0.61), translating to an 18.2-point drop from initial levels. Additionally, this dosage demonstrated statistically notable enhancements across multiple secondary endpoints, such as CGI-S, Marder Factor Scores for Positive and Negative Symptom Change.
"Our Phase 2 dose exploration endeavor successfully pinpointed a daily tolerable dosing regimen, boasting an attractive risk-benefit ratio," commented Eiry W. Roberts, M.D., Neurocrine Biosciences' Chief Medical Officer. "Acknowledging the dire need for innovative schizophrenia therapies, we anticipate advancing NBI-'568, the pioneering M4 selective agonist, into Phase 3 development early next year."
"NBI-1117568 elicited a clinically significant and statistically verified decrease in PANSS scores, accompanied by good tolerability, notably minimal GI disturbances and no weight gain vis-à-vis placebo," remarked Dr. Maurizio Fava, Psychiatrist-in-Chief at Harvard's Massachusetts General Hospital. "As a selective M4 orthosteric agonist, NBI-1117568's potential to alleviate schizophrenia symptoms with fewer adverse effects offers a promising alternative to current treatment options for patients and caregivers."
NBI-'568 demonstrated general safety and tolerability across all tested doses in the Phase 2 trial. Discontinuation rates due to adverse events were comparable between NBI-'568 and placebo groups. Common adverse events included somnolence, dizziness, and headache. Gastrointestinal events like nausea and constipation were infrequent and comparable to placebo. Cardiovascular incidents were rare and clinically insignificant at all doses tested. NBI-'568 did not lead to a significant weight gain compared to placebo. Few extrapyramidal symptom events were reported.
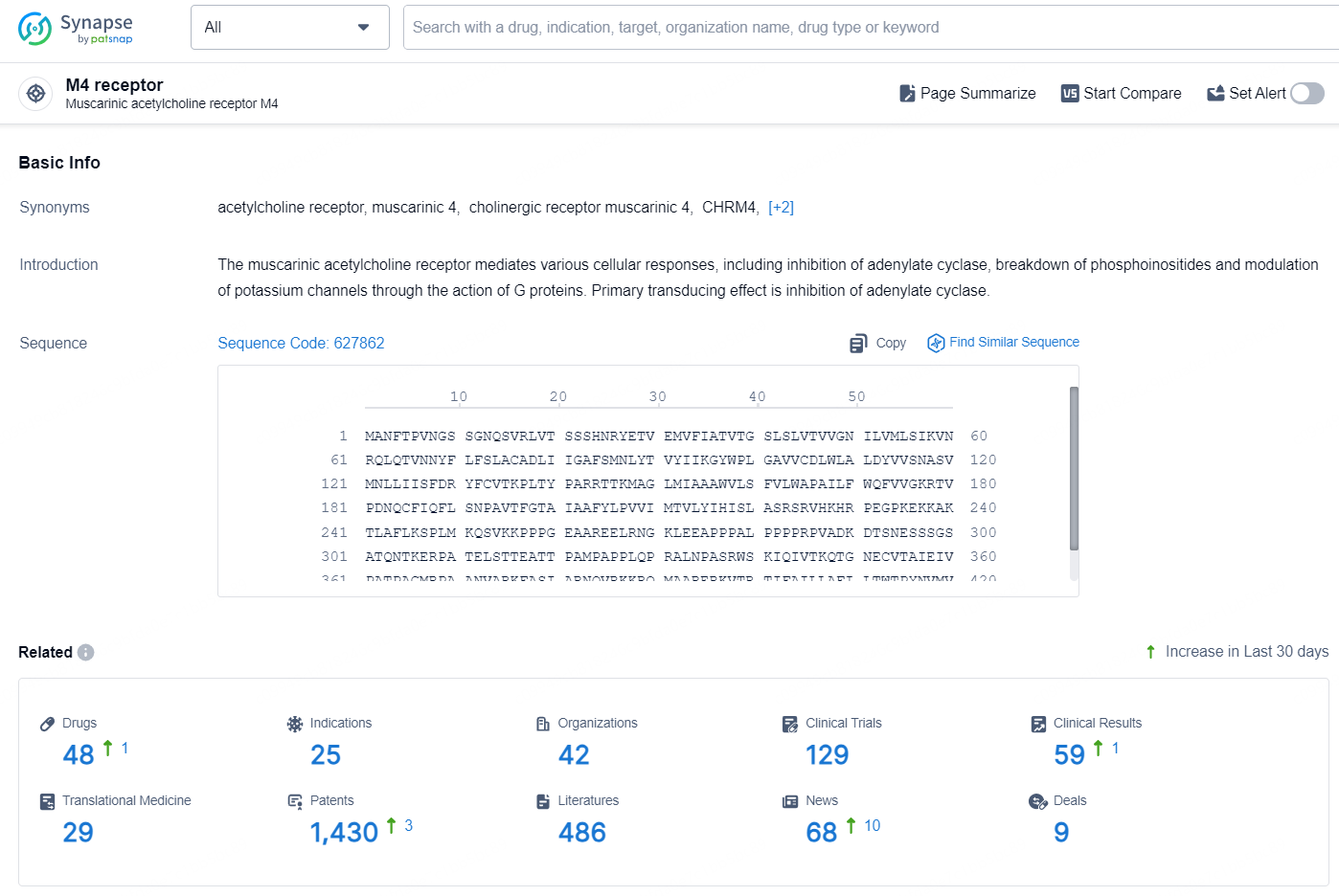
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 2, 2024, there are 48 investigational drugs for the M4 receptor target, including 25 indications, 42 R&D institutions involved, with related clinical trials reaching 129, and as many as 1430 patents.
NBI-1117568 is the first and only M4 selective orthosteric agonist in clinical development. There are five muscarinic acetylcholine receptors involved in neurotransmission. Muscarinic receptors are central to brain function and validated as drug targets in psychosis and cognitive disorders. As an M4 selective orthosteric agonist, NBI-‘568 offers the potential for a novel mechanism with an improved safety profile without the need of combination therapy to minimize off-target pharmacology-related side effects, while also not being dependent on the presence of acetylcholine for efficacy.