Cantargia announces schedules for nadunolimab trials in leukemia and triple negative breast cancer
Cantargia (Cantargia AB, Nasdaq Stockholm: CANTA) has updated the anticipated schedules for nadunolimab clinical studies. The US FDA has issued an IND to MD Andersson Cancer Center for nadunolimab, which pertains to the forthcoming phase 1b/2a clinical trial involving patients diagnosed with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), with trial commencement projected in Q4 2024. Initial data on safety and short-term efficacy from the ongoing phase 2 clinical study in triple negative breast cancer (TNBC), in partnership with GEICAM, is expected in H1 2025.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Göran Forsberg, CEO of Cantargia, expressed, "The approved IND marks a significant milestone in the advancement of nadunolimab for leukemia. We are currently navigating the necessary steps, including IRB review, required to commence the trial." Forsberg continued, "We eagerly anticipate the completion of the Phase 2 study of nadunolimab in triple negative breast cancer and expect to have the results available by the first half of 2025."
The newly proposed phase 1b/2a clinical trial aims to evaluate nadunolimab in up to 20 patients with AML and another 20 with MDS. This trial, funded by a grant from the US Department of Defense (DOD) to The University of Texas MD Anderson Cancer Center, will be executed by the center. Further information regarding the trial, including projected timelines, will be released post-IRB approval, anticipated in Q3 2024, with the trial's commencement expected in Q4 2024.
Building on encouraging phase 1 clinical outcomes for nadunolimab in combination therapy for TNBC, the randomized, controlled phase 2 clinical trial featuring roughly 100 patients with TNBC is ongoing, despite a temporary slowdown in recruitment over the summer. Initial results, encompassing safety and short-term efficacy, are anticipated in the first half of 2025.
Cantargia will unveil new clinical findings involving nadunolimab at the upcoming ESMO conference in Barcelona, scheduled for September 13-17, 2024. Further details of the presentation will be provided when abstracts are made public on September 9, 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
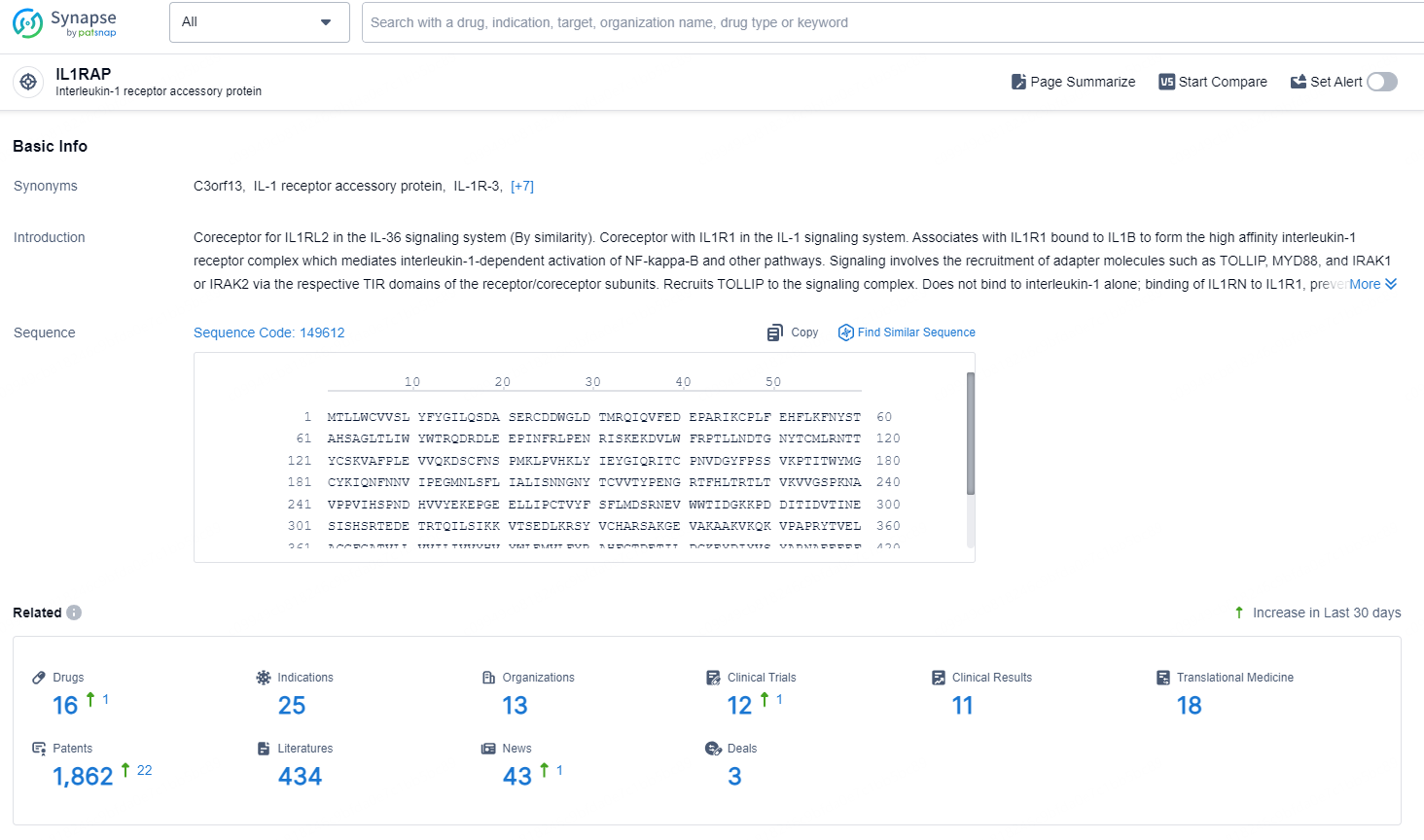
According to the data provided by the Synapse Database, As of August 30, 2024, there are 16 investigational drugs for the IL1RAP targets, including 25 indications, 13 R&D institutions involved, with related clinical trials reaching 12, and as many as 1862 patents.
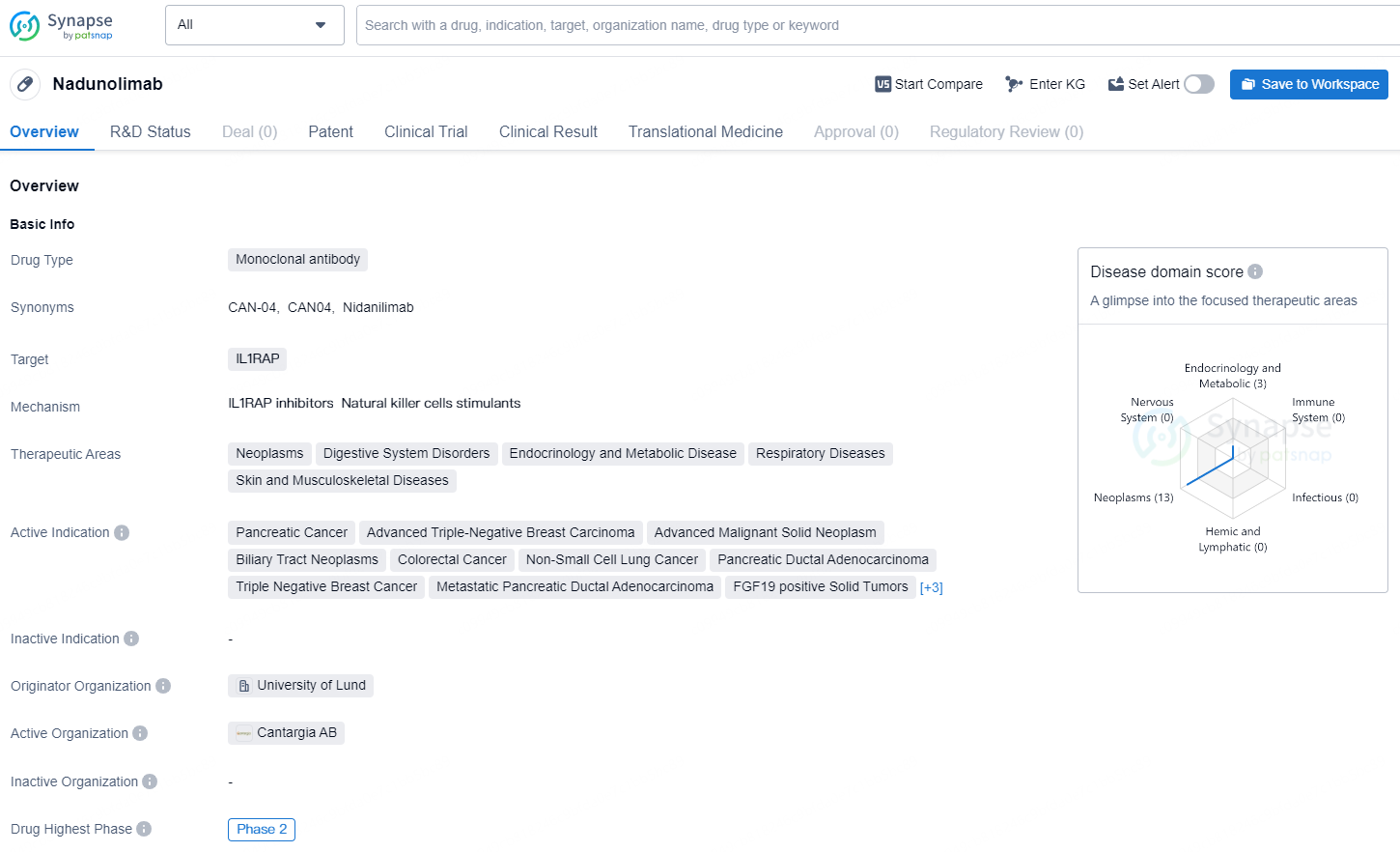
Nadunolimab is a monoclonal antibody drug that targets IL1RAP and is currently in the highest global phase of Phase 2. Its therapeutic areas include neoplasms, digestive system disorders, endocrinology and metabolic disease, respiratory diseases, and skin and musculoskeletal diseases. The drug is indicated for various advanced cancers, including pancreatic cancer, advanced triple-negative breast carcinoma, advanced malignant solid neoplasm, biliary tract neoplasms, colorectal cancer, non-small cell lung cancer, pancreatic ductal adenocarcinoma, triple-negative breast cancer, metastatic pancreatic ductal adenocarcinoma, FGF19 positive solid tumors, melanoma, squamous cell carcinoma of the head and neck, and urothelial carcinoma of the urinary bladder.