Novartis' Leqvio® Significantly Reduces LDL-C in Low to Moderate ASCVD Risk Patients as Standalone Treatment
Novartis reported favorable top-line outcomes from the biannual administration of Leqvio® (inclisiran) in the Phase III V-MONO trial, which successfully met its primary objectives. Leqvio monotherapy demonstrated clinically significant and statistically meaningful reduction in low-density lipoprotein cholesterol (LDL-C) compared to both placebo and ezetimibe in individuals at low or moderate risk for atherosclerotic cardiovascular disease (ASCVD) who were not undergoing lipid-lowering treatment.
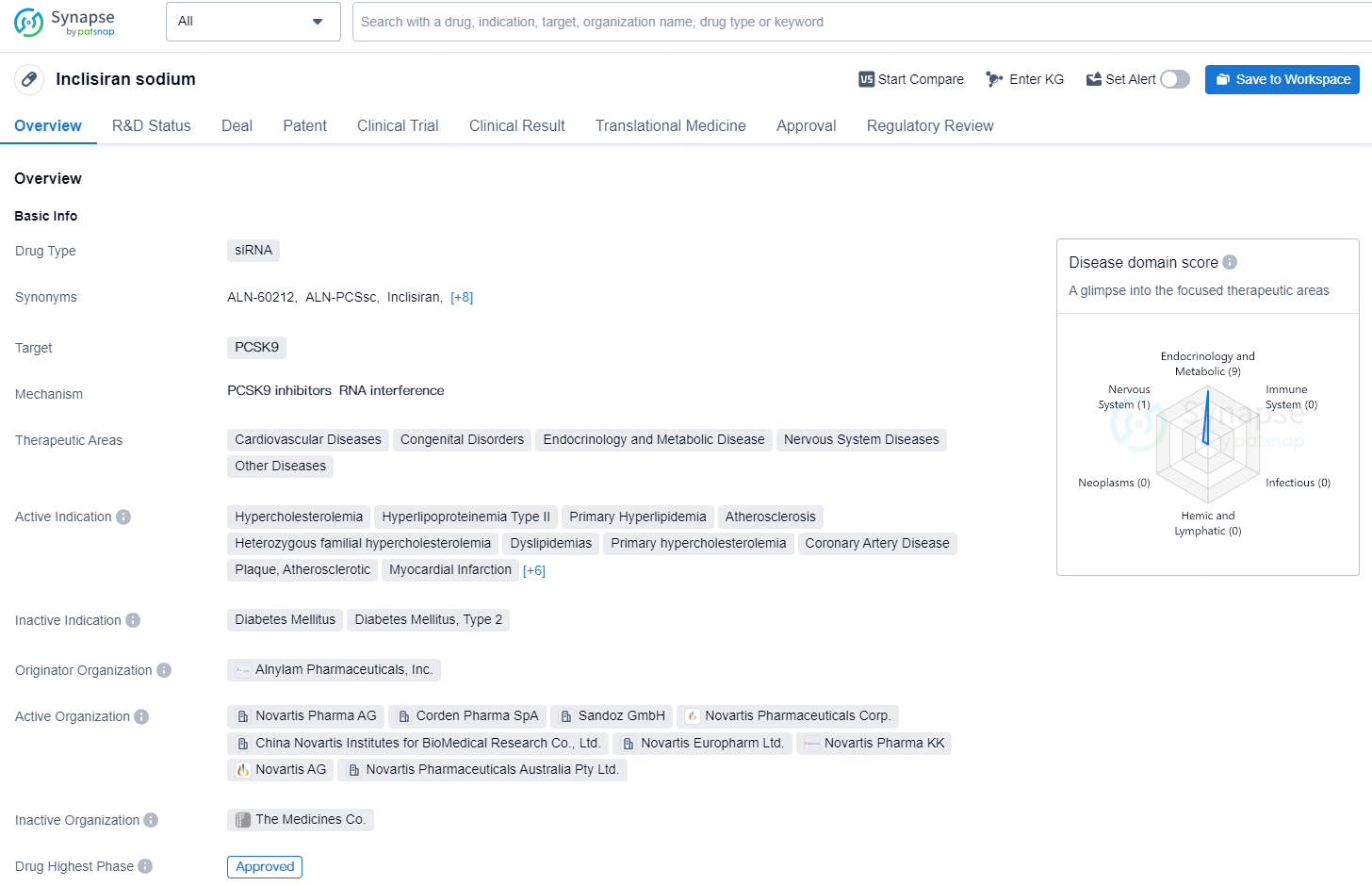
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
V-MONO represents the initial investigation into using a small interfering RNA (siRNA) therapy as a standalone treatment to reduce LDL-C in individuals with a low to moderate risk of developing ASCVD. Novartis is set to present findings from this study at an upcoming medical conference and will share the data with regulatory bodies including the US Food and Drug Administration (FDA).
“We are honored to continue pushing the boundaries of scientific knowledge regarding siRNA therapy to address one of the major global healthcare issues, as many individuals still find it challenging to achieve their cholesterol targets,” stated Shreeram Aradhye, M.D., President, Development and Chief Medical Officer, Novartis. “This study contributes to the expanding evidence base for Leqvio across the complete ASCVD risk spectrum, as we aim to assist more patients in need.”
Novartis is further progressing several studies examining Leqvio's potential applications for both primary and secondary prevention. The VICTORION-1-PREVENT (V1P) study is uniquely focused on a high-risk primary prevention population, as identified by guidelines from the American College of Cardiology (ACC) and American Heart Association (AHA); this outcomes study is anticipated to finish enrollment by the end of this year2. In the context of secondary prevention, the ORION-4 and VICTORION-2-PREVENT (V2P) outcomes studies are on schedule for data releases in 2026 and 2027, respectively.
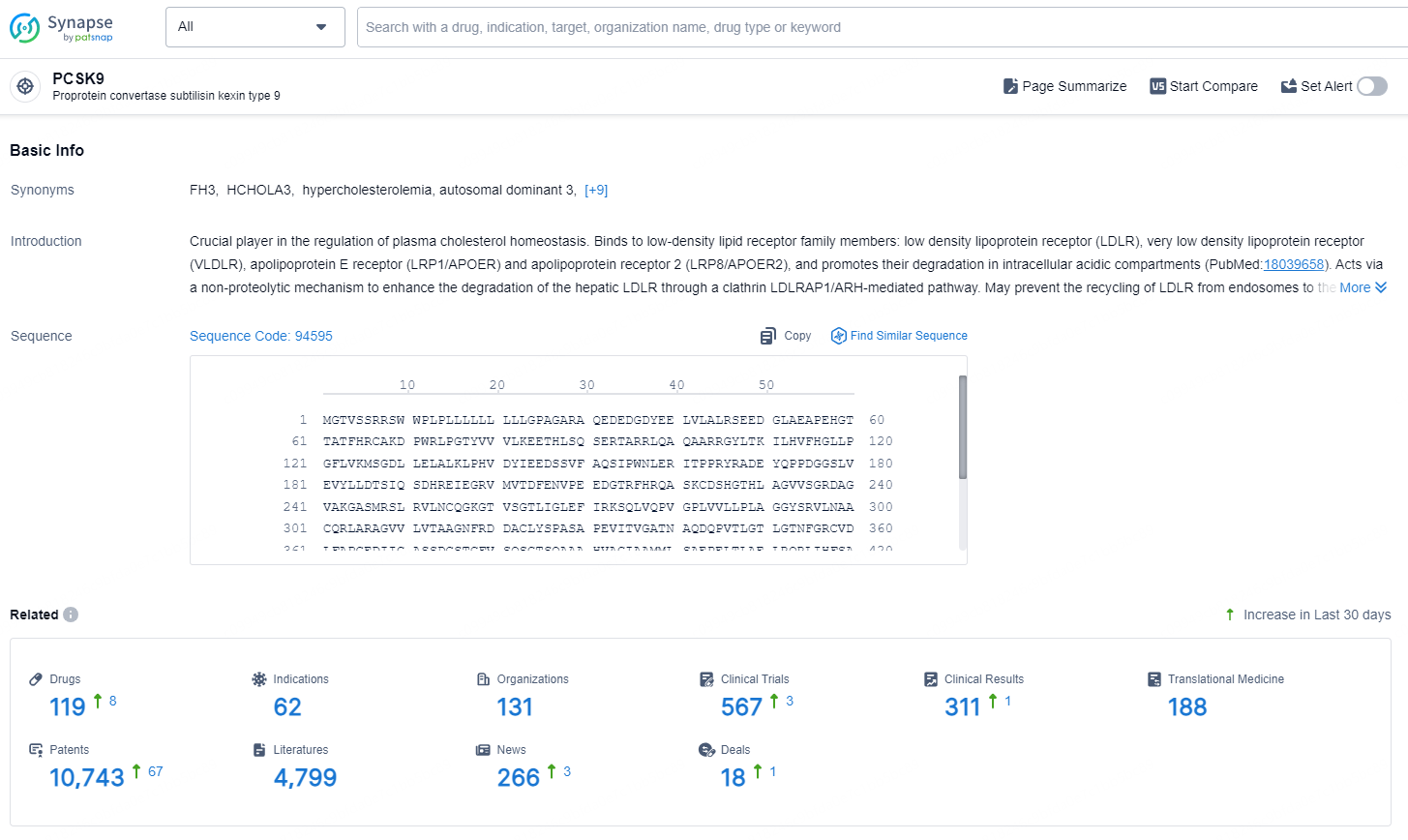
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 30, 2024, there are 119 investigational drugs for the PCSK9 targets, including 62 indications, 131 R&D institutions involved, with related clinical trials reaching 567, and as many as 10743 patents.
Inclisiran sodium is a siRNA-based drug that targets PCSK9 and is indicated for the treatment of a wide range of cardiovascular diseases, congenital disorders, endocrinology and metabolic diseases, nervous system diseases, and other diseases. The drug is specifically indicated for conditions such as hypercholesterolemia, hyperlipoproteinemia Type II, primary hyperlipidemia, atherosclerosis, heterozygous familial hypercholesterolemia, dyslipidemias, primary hypercholesterolemia, coronary artery disease, plaque, atherosclerotic, myocardial infarction, peripheral arterial disease, stroke, hyperlipoproteinemias, acute coronary syndrome, and homozygous familial hypercholesterolemia.