Is Capmatinib approved by the FDA?
Capmatinib, marketed under the brand name Tabrecta, is indeed FDA approved. The FDA granted approval for capmatinib on May 6, 2020. Capmatinib is used to treat non-small cell lung cancer (NSCLC) that has spread to other parts of the body (metastatic) or cannot be removed with surgery. This approval specifically targets adult patients with tumors exhibiting a mutation that leads to mesenchymal-epithelial transition (MET) exon 14 skipping, as detected by an FDA-approved test.
What is Capmatinib?
Capmatinib is an oral medication belonging to the drug class known as multikinase inhibitors. It is available in tablet form, with dosages of 150 mg and 200 mg. This targeted therapy works by inhibiting the MET receptor tyrosine kinase, which plays a critical role in oncogenic signaling pathways.
Dosage and Administration
The usual adult dose for treating NSCLC is 400 mg taken orally twice daily. Patients can take capmatinib with or without food, but it is essential to follow the prescription label and any additional instructions provided by healthcare providers. Regular blood tests are necessary to monitor liver function during treatment.
Side Effects
Common side effects of capmatinib include trouble breathing, nausea, vomiting, decreased appetite, fatigue, abnormal liver function tests, and swelling in the hands or feet. Serious side effects that require immediate medical attention include:
- New or worsening cough, chest pain, or trouble breathing
- Fever, cough with mucus
- Severe ongoing nausea and vomiting
- Signs of liver or pancreas problems such as loss of appetite, upper stomach pain, jaundice, dark urine, or a fast heart rate
Patients should report any side effects to their doctor and can also contact the FDA at 1-800-FDA-1088.
Warnings and Precautions
Before taking capmatinib, patients should inform their doctors of any existing lung conditions other than lung cancer and any history of liver disease. Women who are pregnant or breastfeeding should avoid using capmatinib due to potential harm to the unborn baby. Both men and women should use effective birth control during treatment and for at least one week after the last dose.
Capmatinib can make patients more susceptible to sunburn; therefore, they should avoid excessive sunlight or tanning beds and use sunscreen with an SPF of 30 or higher when outdoors.
Drug Interactions
Capmatinib can interact with other medications, including prescription and over-the-counter medicines, vitamins, and herbal products. It is crucial to inform healthcare providers about all current medications to avoid adverse interactions.
Conclusion
Capmatinib, under the brand name Tabrecta, is FDA-approved for the treatment of metastatic non-small cell lung cancer with a specific MET gene mutation. This approval provides a significant treatment option for patients with this particular genetic profile, advancing the approach to personalized cancer therapy.
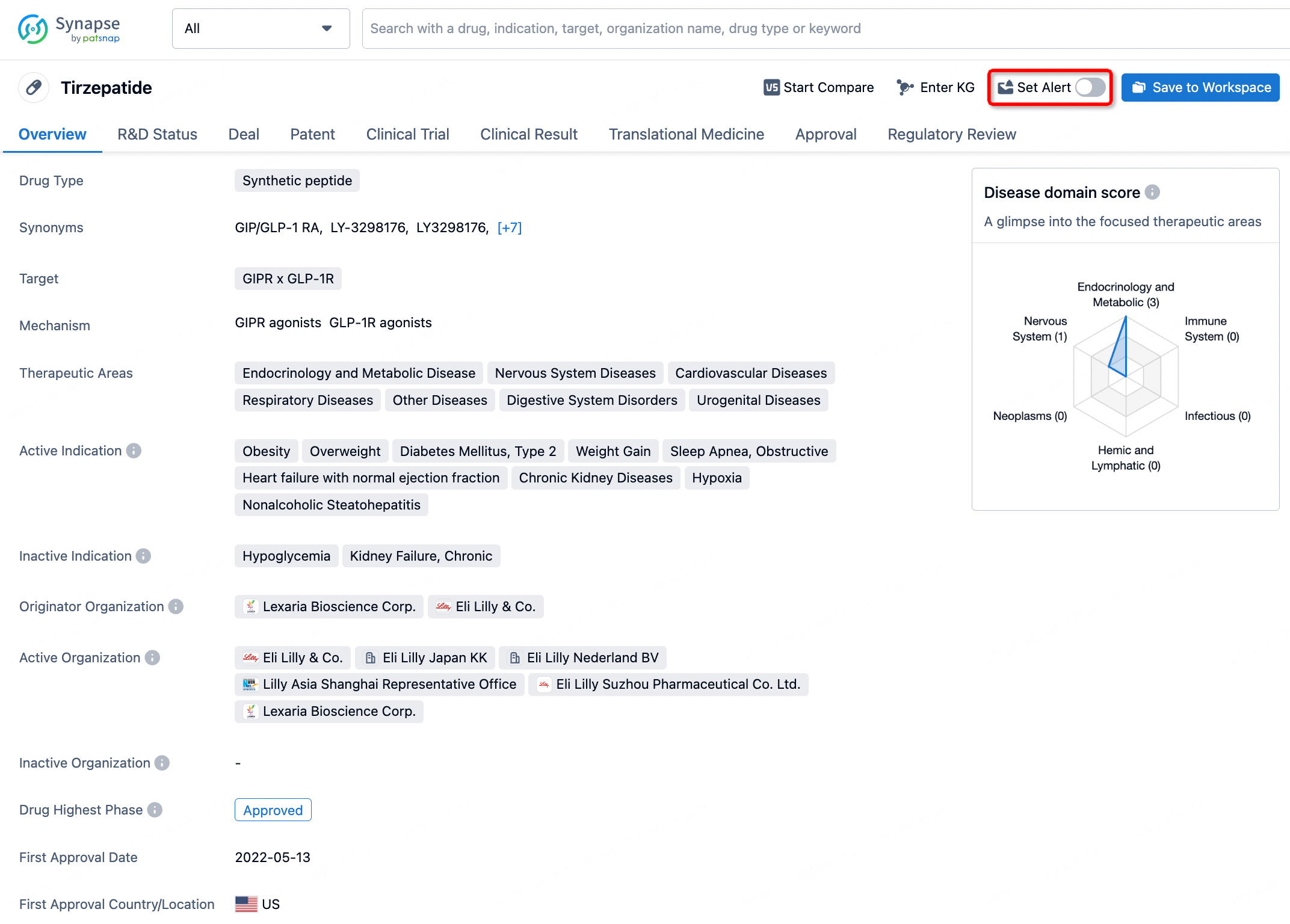
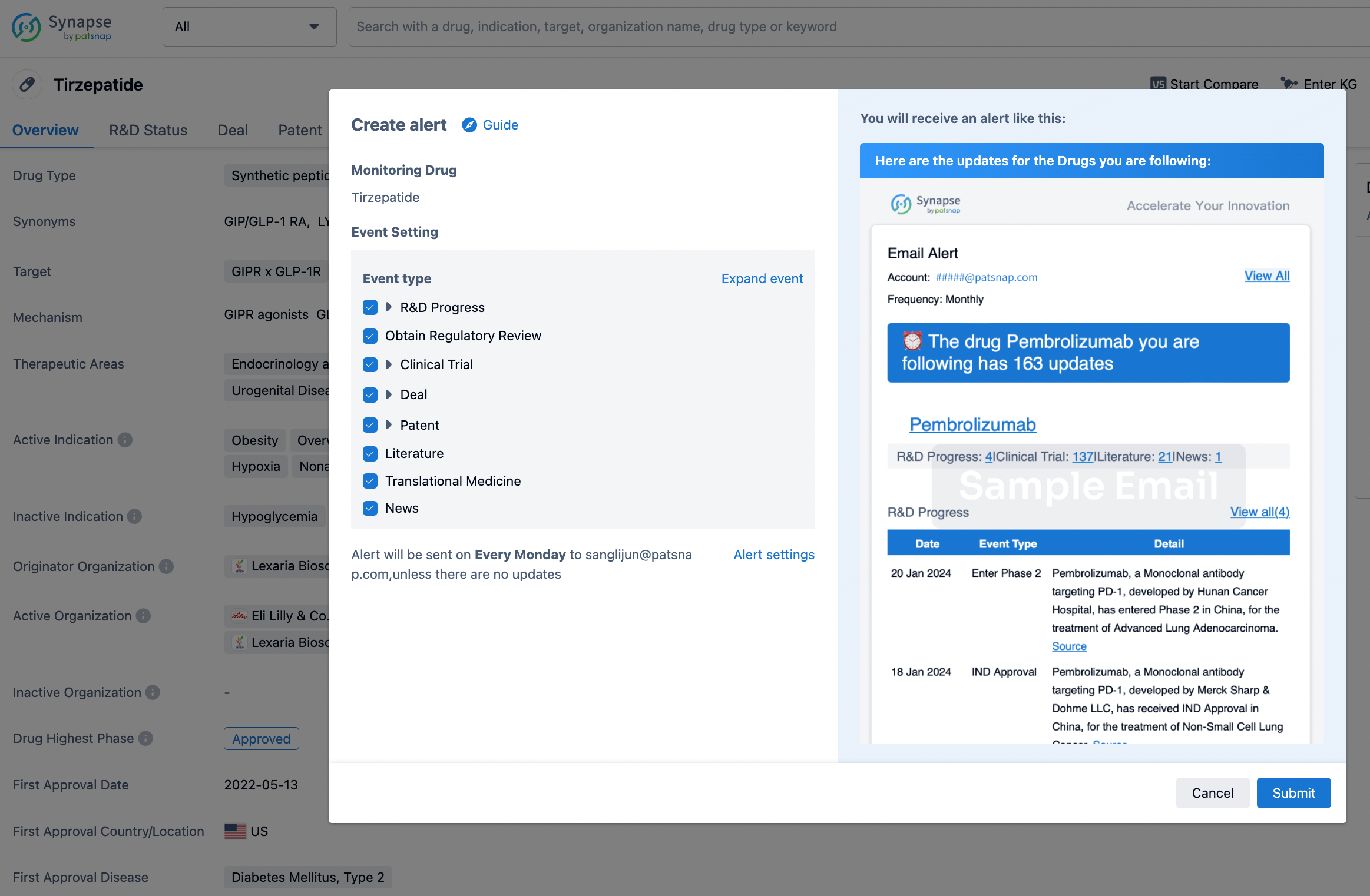
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!