Enlivex Authorized for Phase I Trial of Allocetra in Psoriatic Arthritis Patients
Enlivex Therapeutics Ltd., a company focused on clinical-stage immunotherapy through macrophage reprogramming, announced today that the Israeli Ministry of Health has approved the start of a Phase I clinical trial. This trial aims to assess the safety and tolerability of Allocetra™ when injected into the joints of patients suffering from psoriatic arthritis.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The trial is currently set to enroll six patients who have shown an inadequate response to standard treatments for psoriatic arthritis. The primary safety endpoint will observe the frequency and severity of adverse events and serious adverse events. Secondary endpoints will evaluate changes from baseline in pain and other disease activity parameters for up to 12 months post-administration of Allocetra™.
“There are numerous patients with psoriatic arthritis who do not respond well to currently available therapies. Allocetra™'s mode of action may offer a new approach to treating these patients. We are excited to announce the start of a new clinical program focusing on psoriatic arthritis and are eager to begin the study, marking our initial step toward potentially developing an effective treatment option for these individuals,” stated Oren Hershkovitz, Ph.D., CEO of Enlivex.
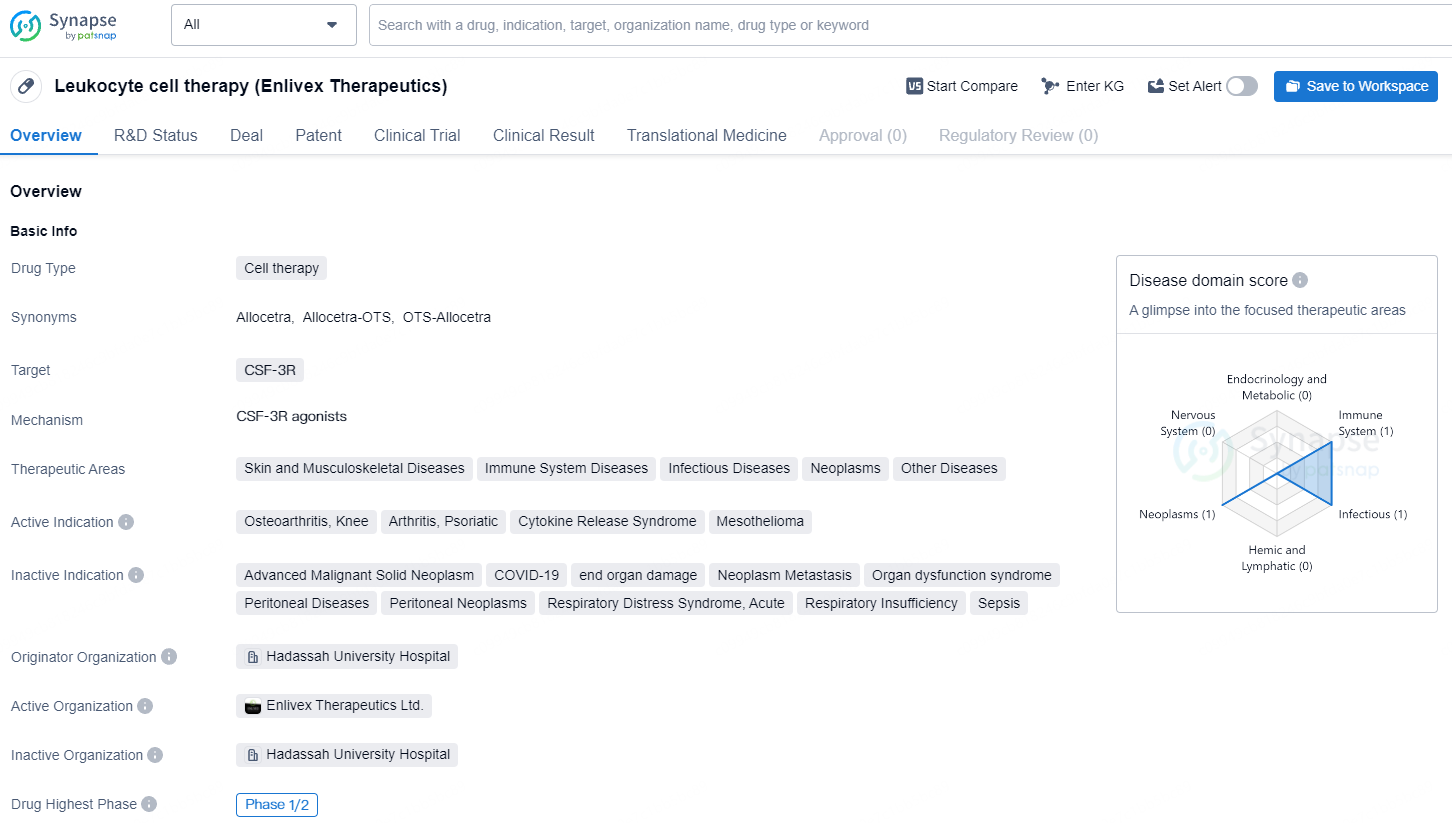
Allocetra™ is being developed as a universal, off-the-shelf cell therapy intended to reprogram macrophages back to their homeostatic state. Conditions such as sepsis, osteoarthritis, and psoriatic arthritis, among others, cause macrophages to lose their homeostatic state, significantly contributing to disease severity.
By restoring macrophage homeostasis and leveraging their healing capabilities, Allocetra™ holds promise as a novel immunotherapeutic approach for critical and debilitating clinical indications classified as “unmet medical needs.” It can function as a stand-alone therapy or in conjunction with leading therapeutic agents.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
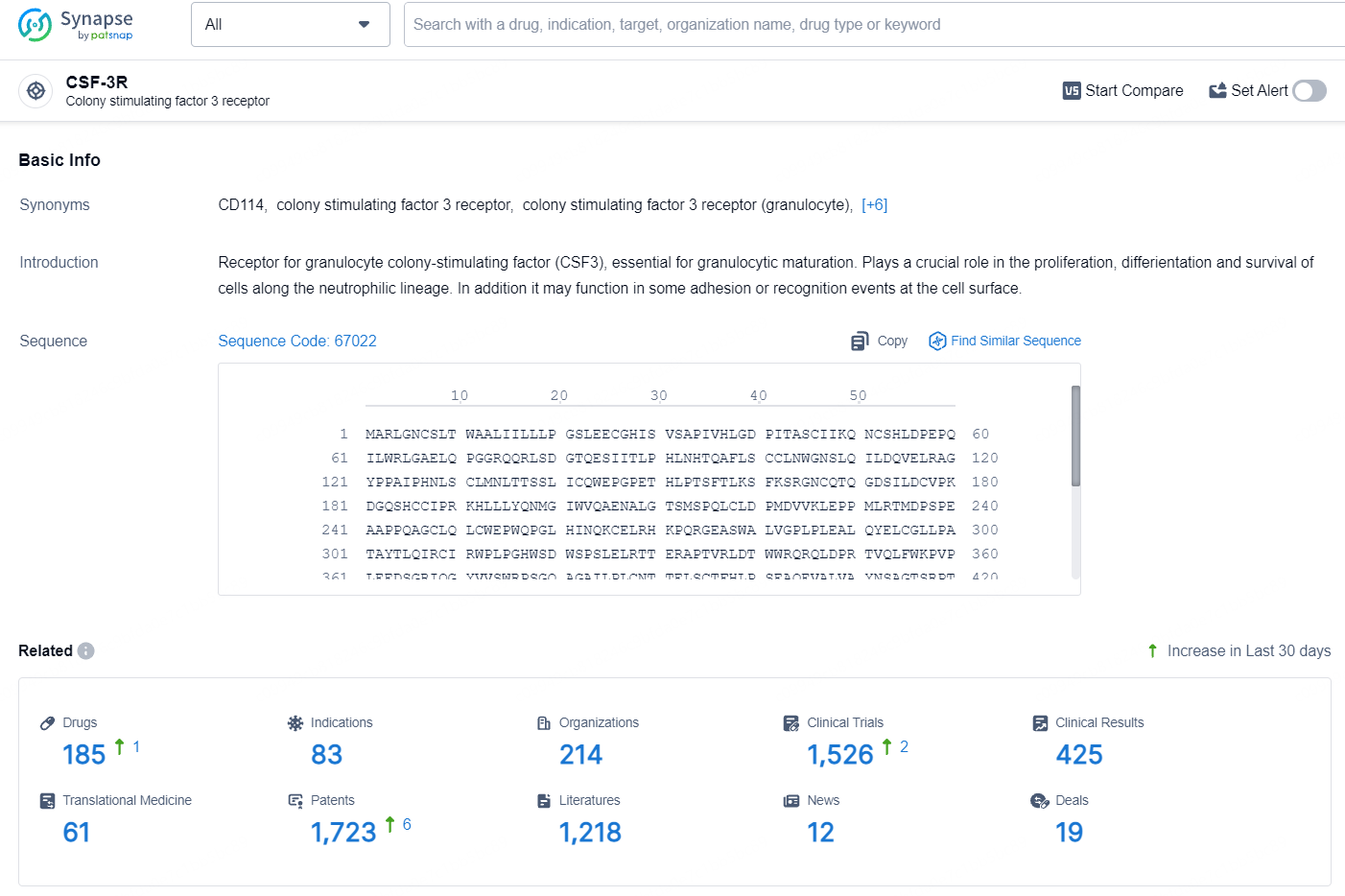
According to the data provided by the Synapse Database, As of July 30, 2024, there are 185 investigational drugs for the CSF-3R target, including 83 indications, 214 R&D institutions involved, with related clinical trials reaching 1526, and as many as 1723 patents.
Leukocyte cell therapy is a type of cell therapy that targets CSF-3R. The therapeutic areas for this drug are focused on skin and musculoskeletal diseases, neoplasms, and other diseases. The active indications for this drug include osteoarthritis of the knee, cytokine release syndrome, and mesothelioma. The drug has reached Phase 1/2 of development, indicating that it has shown promise in early clinical trials and is progressing towards further evaluation and potential approval.