Is Adstiladrin approved by the FDA?
Adstiladrin, with the generic name nadofaragene firadenovec-vncg, is a novel gene therapy for treating a specific type of bladder cancer. Adstiladrin received FDA approval on December 16, 2022. It represents a significant advancement in cancer therapy, particularly for patients who have not responded to standard treatments.
What is Adstiladrin?
Adstiladrin is a gene therapy product that delivers the gene for interferon alfa-2b directly into the bladder cells. This gene therapy is based on a non-replicating adenovirus vector, which means the virus used to deliver the gene does not multiply within the body. Instead, it inserts the interferon alfa-2b gene into the cells of the bladder wall, causing them to produce high levels of interferon alfa-2b, a protein that helps fight cancer.
How is Adstiladrin Used?
Adstiladrin is administered intravesically, meaning it is directly instilled into the bladder through a urinary catheter. This treatment is given once every three months. The typical dose is 75 mL at a concentration of 3 x 10^11 viral particles per mL. During the treatment, the patient needs to retain the solution in the bladder for one hour, during which they may be moved every 15 minutes to ensure even distribution.
Usage Instructions
After each treatment, specific steps must be followed to safely handle urination, due to the presence of the gene therapy in the urine. Patients should:
- Add half a cup of bleach to the toilet bowl before urinating.
- Wait 15 minutes before flushing.
- Repeat these steps for the first two days following treatment.
Precautions and Contraindications
Adstiladrin is not suitable for everyone. It should not be used by individuals who are immunosuppressed or have immune deficiencies. Additionally, those who have a sensitivity to interferon alfa or any of its components should avoid this treatment. Pregnant or breastfeeding women should also discuss potential risks with their healthcare provider.
Side Effects
The most common side effects of Adstiladrin include:
- Urinary discharge
- Fatigue
- Bladder spasms
- Urgency to urinate
- Hematuria (blood in the urine)
Patients are advised to report any side effects to their healthcare provider or directly to the FDA.
Conclusion
Adstiladrin offers a promising new treatment for patients with high-risk NMIBC that has not responded to BCG. Its FDA approval on December 16, 2022, provides an innovative option in the fight against bladder cancer, utilizing gene therapy to deliver potent anticancer effects directly to the site of the tumor. This advancement underscores the potential of gene therapy in treating complex cancers and improving patient outcomes.
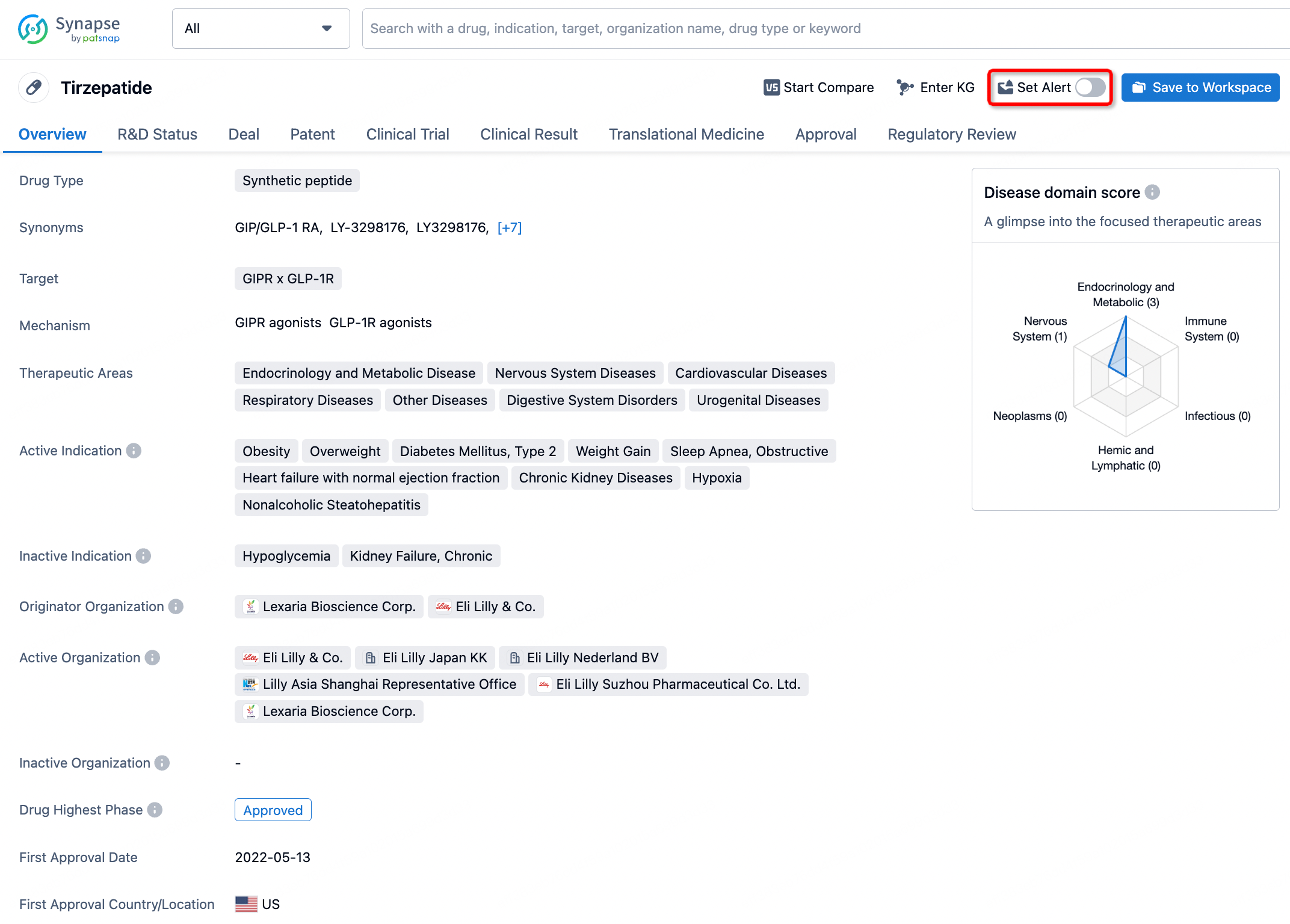
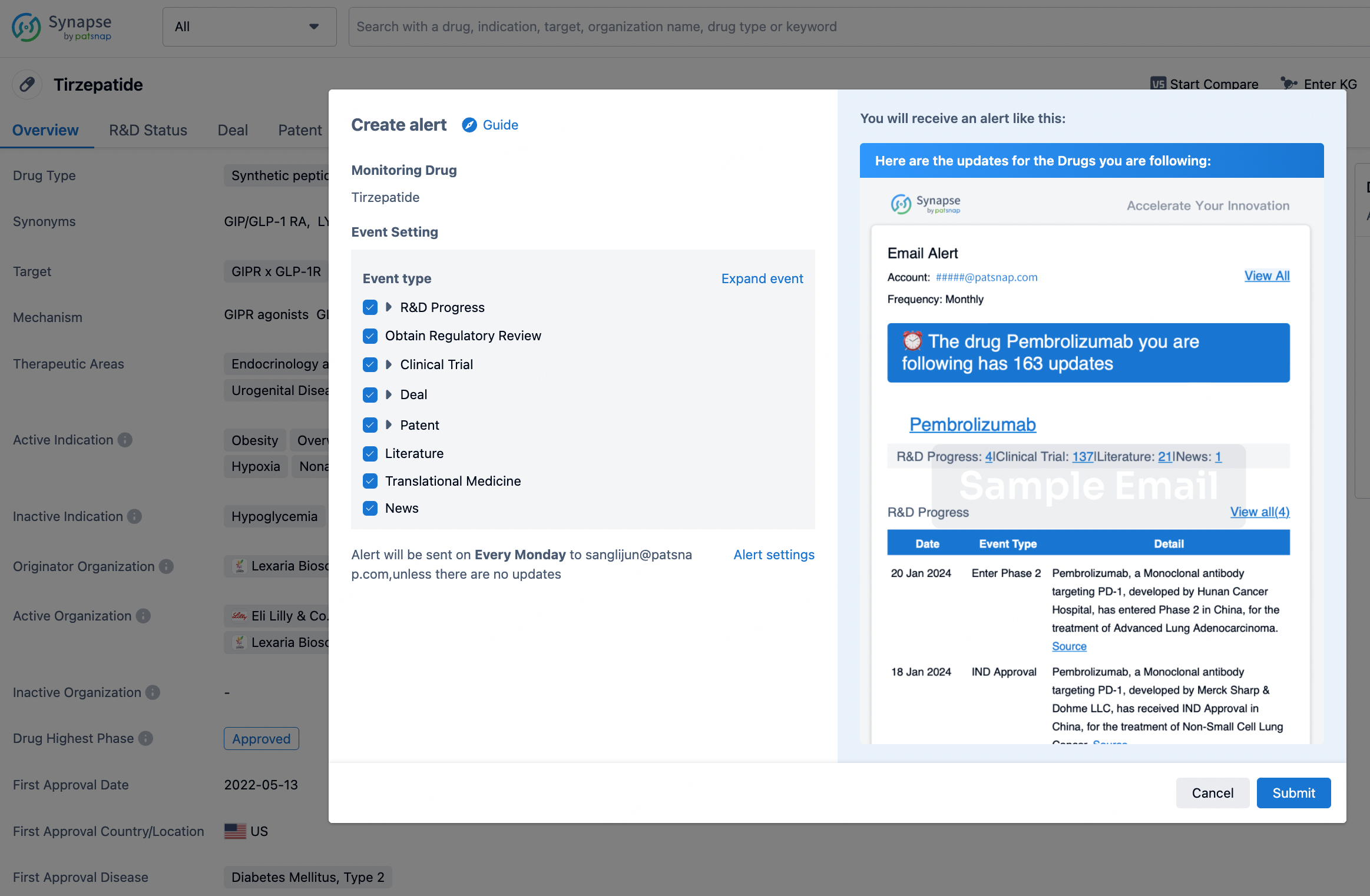
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!