Promising Phase 2 Results for OSE Immunotherapeutics' Lusvertikimab in Treating Ulcerative Colitis
OSE Immunotherapeutics SA announced initial positive outcomes from its CoTikiS randomized, double-blind, placebo-controlled, Phase 2 Proof of Concept trial of Lusvertikimab, an IL-7 receptor pure antagonist, showing notable efficacy results as evidenced by the enhancement of the Modified Mayo Score.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 A positive safety profile was identified during both the induction and 6-month open-label extension phases of the trial. This clinical proof of concept study validates Lusvertikimab as a promising first-in-class therapy, thanks to its novel mechanism of action as an exclusive interleukin-7 antagonist.
A positive safety profile was identified during both the induction and 6-month open-label extension phases of the trial. This clinical proof of concept study validates Lusvertikimab as a promising first-in-class therapy, thanks to its novel mechanism of action as an exclusive interleukin-7 antagonist.
Nicolas Poirier, CEO of OSE Immunotherapeutics, remarked: "We are thrilled to announce these initial positive Phase 2 efficacy results for Lusvertikimab in treating ulcerative colitis, a challenging chronic relapsing inflammatory bowel disease with a high demand for new therapeutic options. We hold great hope for patients, as these positive clinical efficacy and safety outcomes serve as a strong catalyst for future opportunities, enhancing OSE's position in the burgeoning field of chronic immune inflammation."
Frédérique Corallo, CMO Immuno-Inflammation, stated: "We are profoundly grateful to the patients who took part in this trial, as well as to the study investigators and the global team for their unwavering dedication to achieving this significant clinical milestone."
"Lusvertikimab has demonstrated very promising efficacy results with the two doses tested at week 10, showing significant endoscopic improvement, with sustained efficacy signals observed for 34 weeks during the open-label extension. A positive safety profile was noted in the entire patient population. The full dataset will be disseminated in a subsequent communication and presented at upcoming medical conferences," she added.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
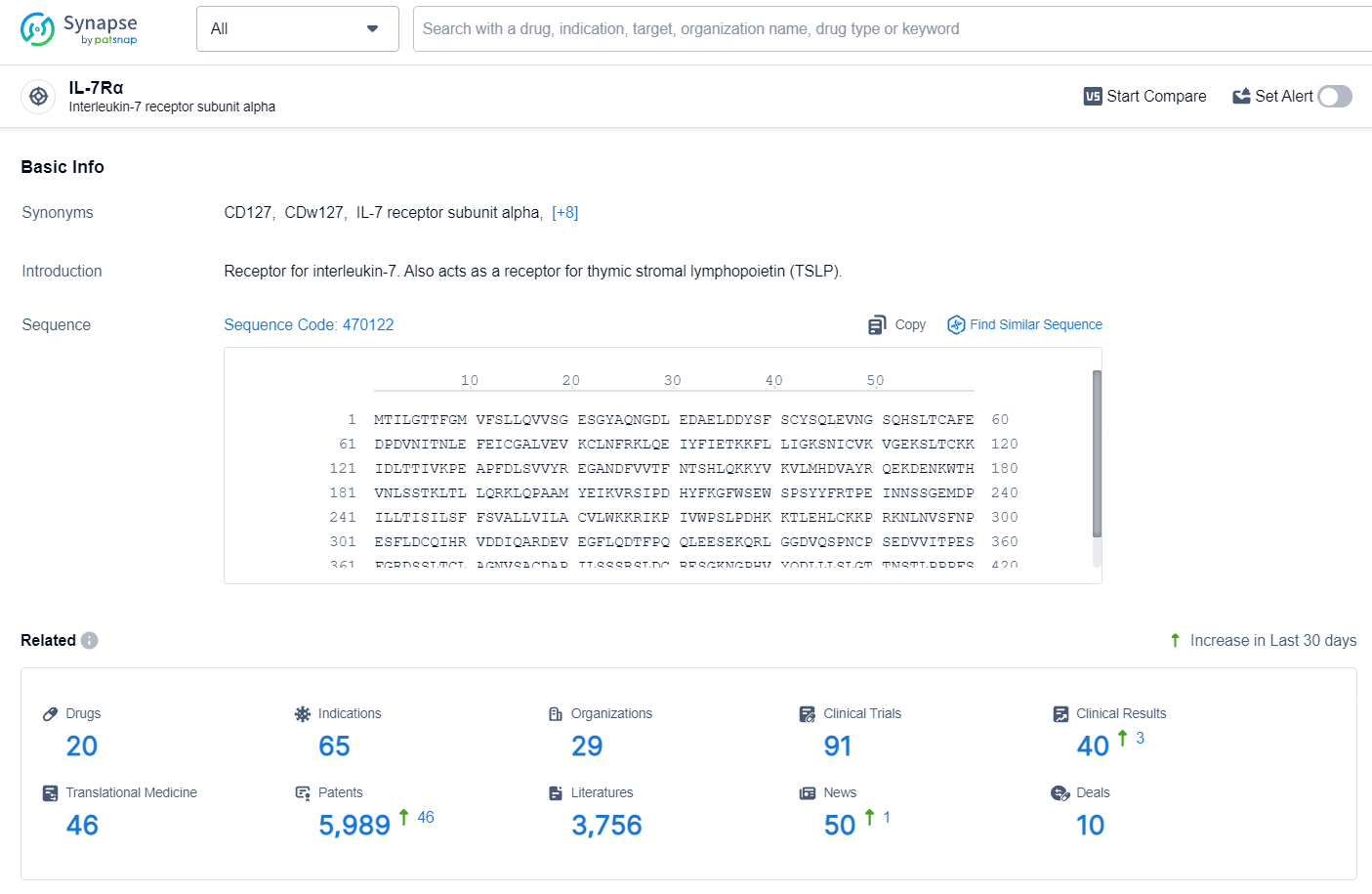
According to the data provided by the Synapse Database, As of July 25, 2024, there are 20 investigational drugs for the IL-7 targets, including 65 indications, 29 R&D institutions involved, with related clinical trials reaching 91, and as many as 5989 patents.
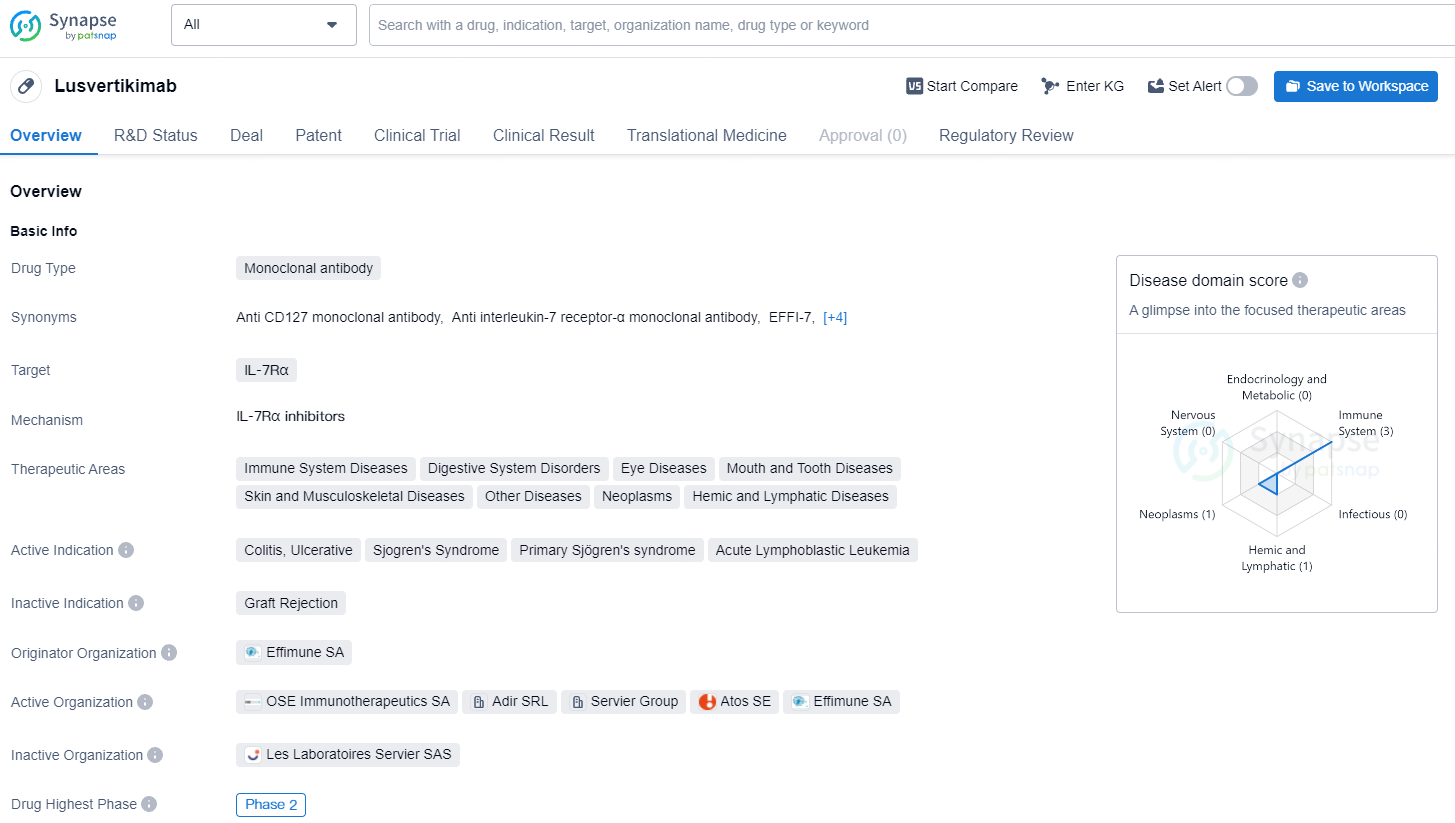
Lusvertikimab elucidates its potential as a treatment for various conditions, indicating a broad spectrum of therapeutic use. The drug's current development phase and regulatory status highlights its progression within the pharmaceutical industry, as it moves towards potentially providing solutions for unmet medical needs in the field of biomedicine.