Erdafitinib: A Quick Look at Its R&D Progress and Clinical Results from the 2024 ASCO_GU
TAR-210 is an intravesical drug delivery system that is designed to provide local, continuous release of erdafitinib. Its safety and efficacy are being evaluated in a first-in-human clinical study (NCT05316155) of patients with bladder cancer whose tumors harbor select FGFR alterations (alt). Toovercome tissue-based challenges in identifying susceptible FGFRalt to select patients for treatment with TAR-210, Janssen partnered with Predicine to use a proprietary urine cell-free DNA diagnostic assay (PredicineCARE). Validation of the urine assay to detect FGFRalt was previously demonstrated using urine samples collected in a highly controlled manner via a collaboration with Stratifyer Molecular Pathology GmbH, Cologne, Germany (Kim et al. ASCO GU 2023). However, evaluation of the urine assay for diagnostic screening in a real-world setting is currently lacking. Recently, the preliminary results of the urine assay for patient selection and the characterization of the urine-defined genomic landscape in screened patients were reported in the 2024 ASCO_GU.
Erdafitinib's R&D Progress
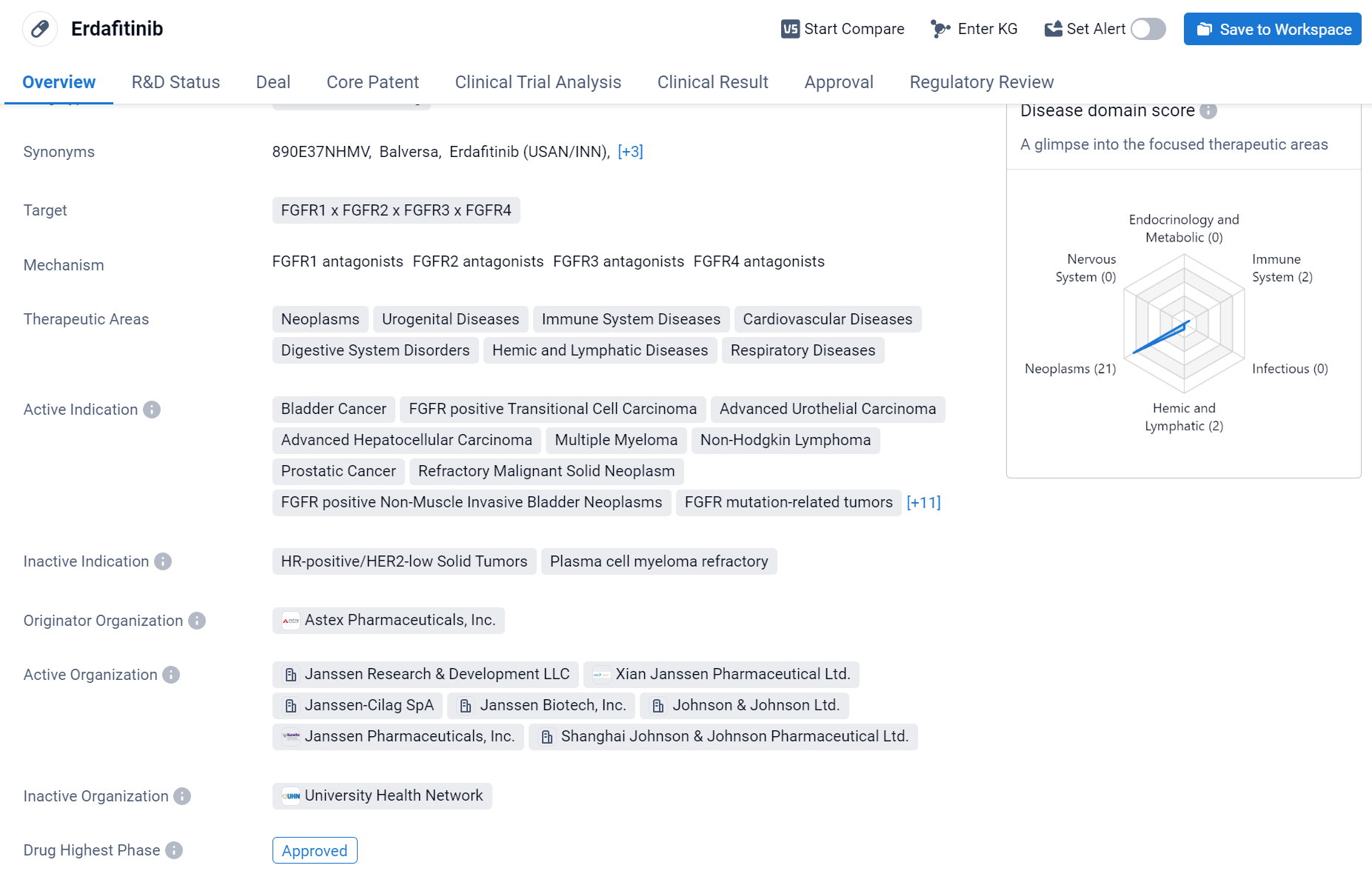
Erdafitinib is a small molecule drug that targets FGFR1, FGFR2, FGFR3, and FGFR4. It has been developed by Astex Pharmaceuticals, Inc. and has received approval for use in several therapeutic areas, including neoplasms, urogenital diseases, immune system diseases, cardiovascular diseases, digestive system disorders, hemic and lymphatic diseases, and respiratory diseases.
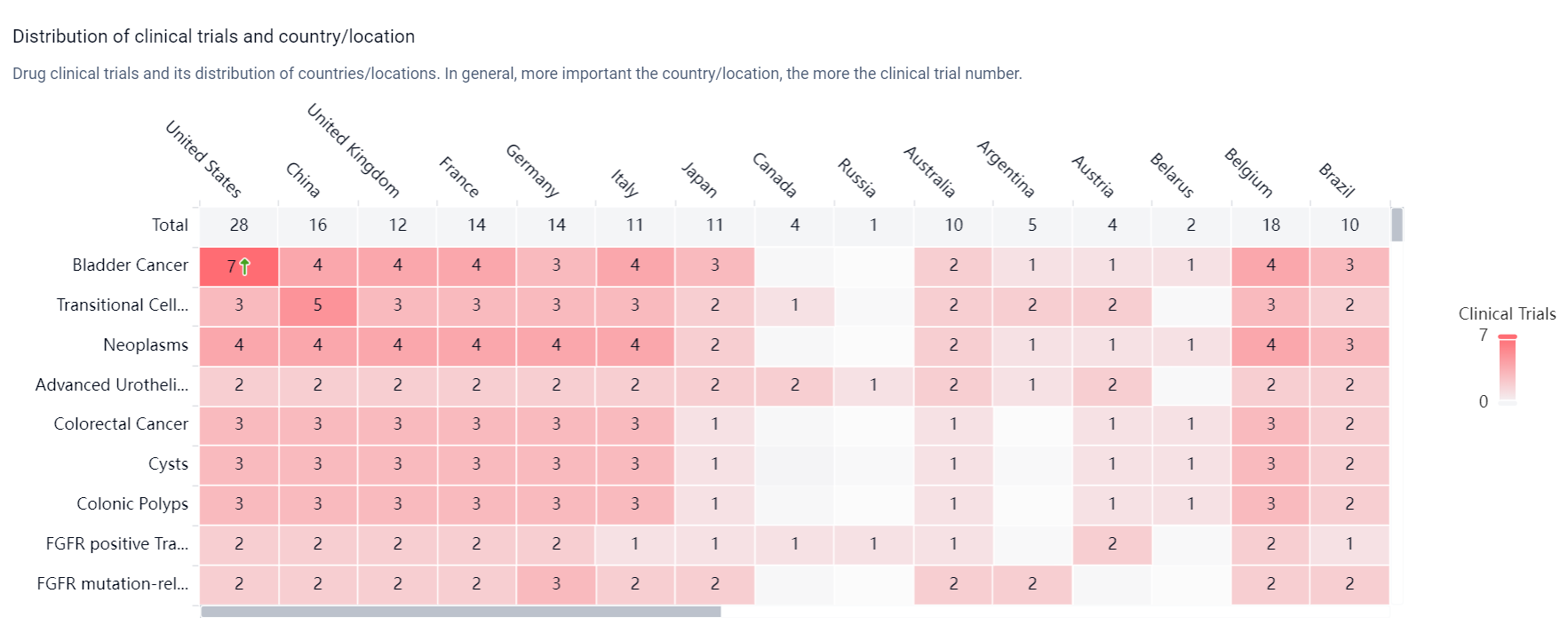
According to the Patsnap Synapse, Erdafitinibis currently in the highest phase of development globally, with NDA/BLA status in China. And the clinical trial distributions for Erdafitinib are primarily in the United States, China and United Kingdom. The key indication is Bladder Cancer.
Detailed Clinical Result of Erdafitinib
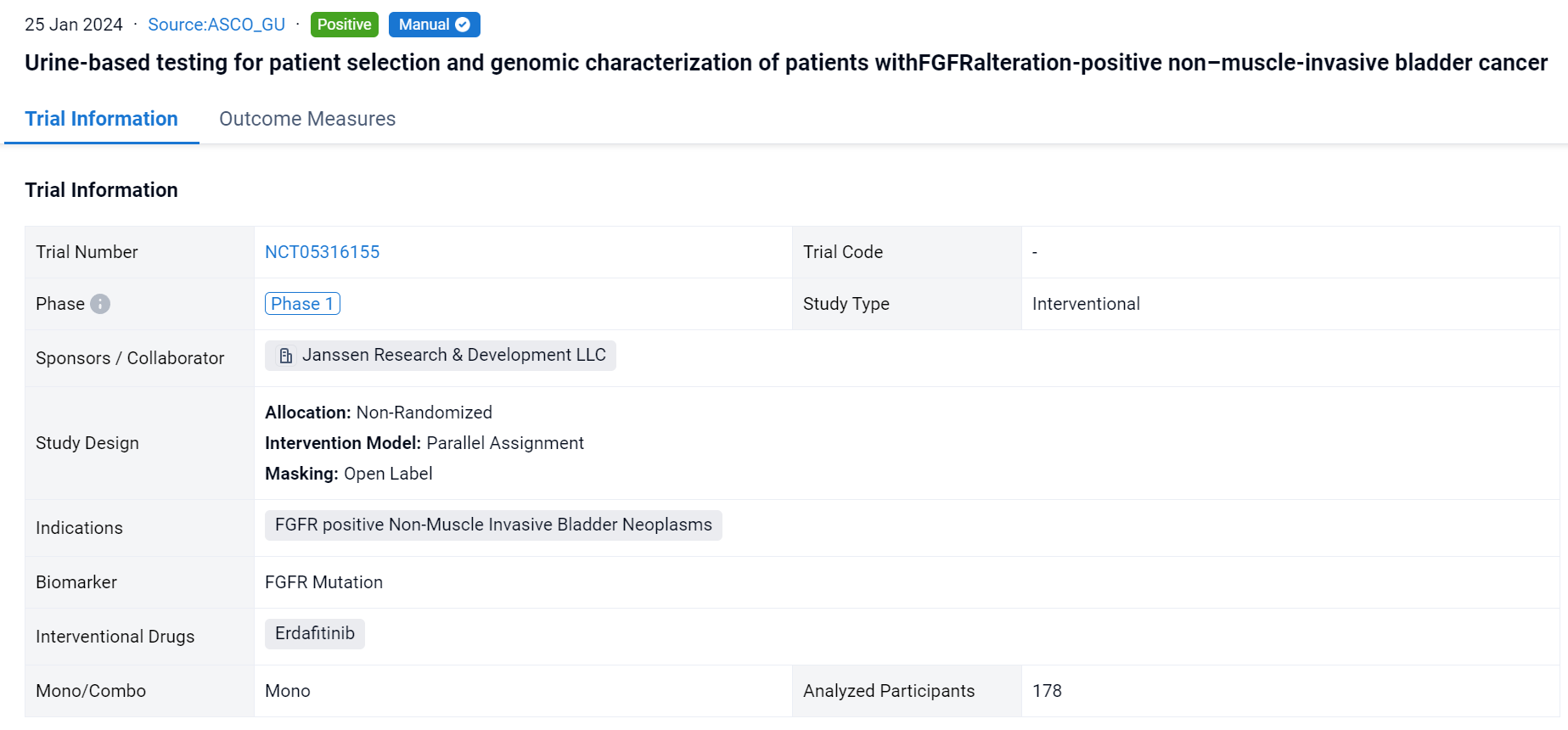
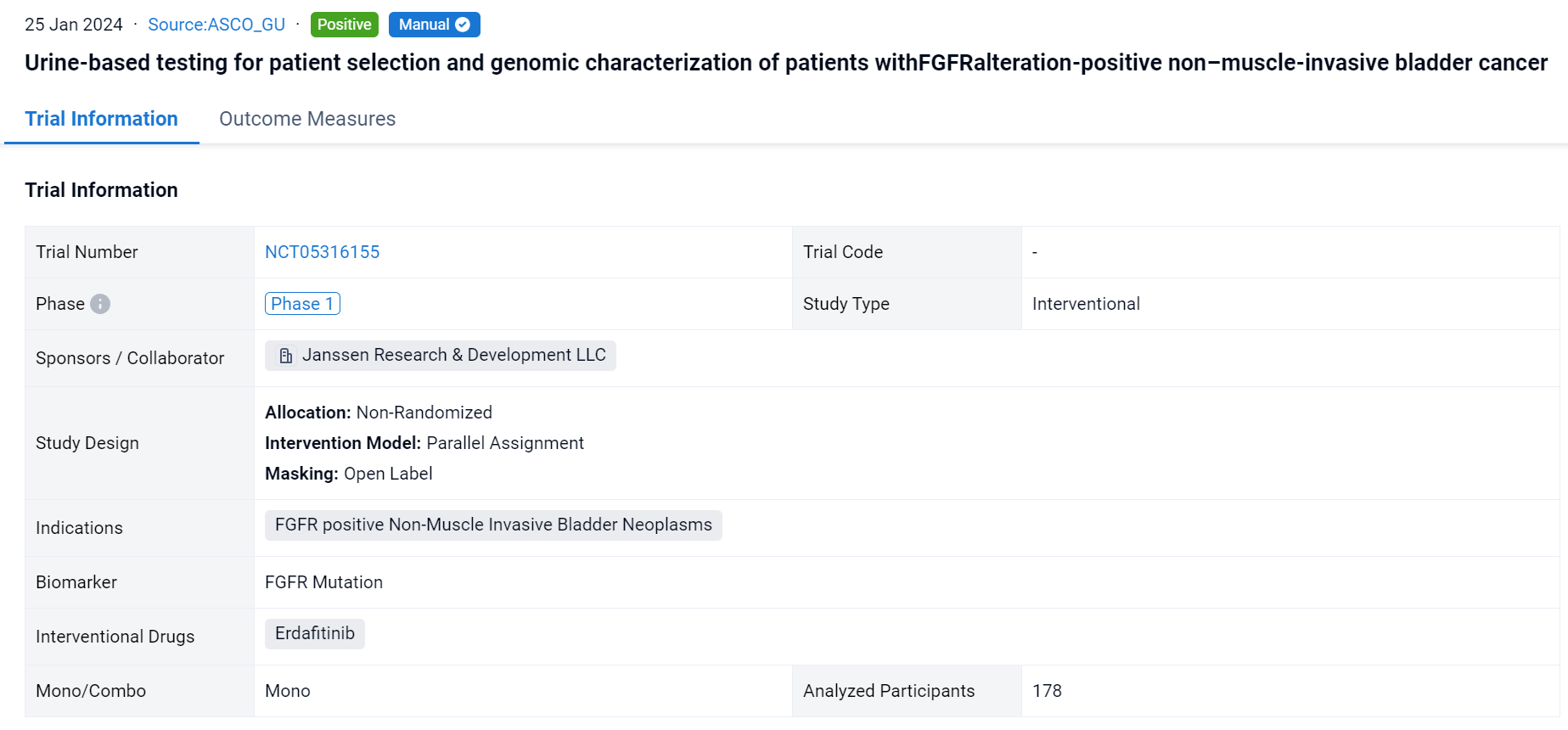
This non-randomized, parallel assignment, open-labeled clinical trial (NCT05316155) was aimed to evaluate the urine assay for diagnostic screening in a real-world setting.
In this study, Enrollment was based on detection of prespecified FGFRalt from either tumor tissue obtained from previous biopsies (Qiagen Therascreen FGFR assay) or urine samples obtained prior to enrollment (PredicineCARE next-generation sequencing assay).
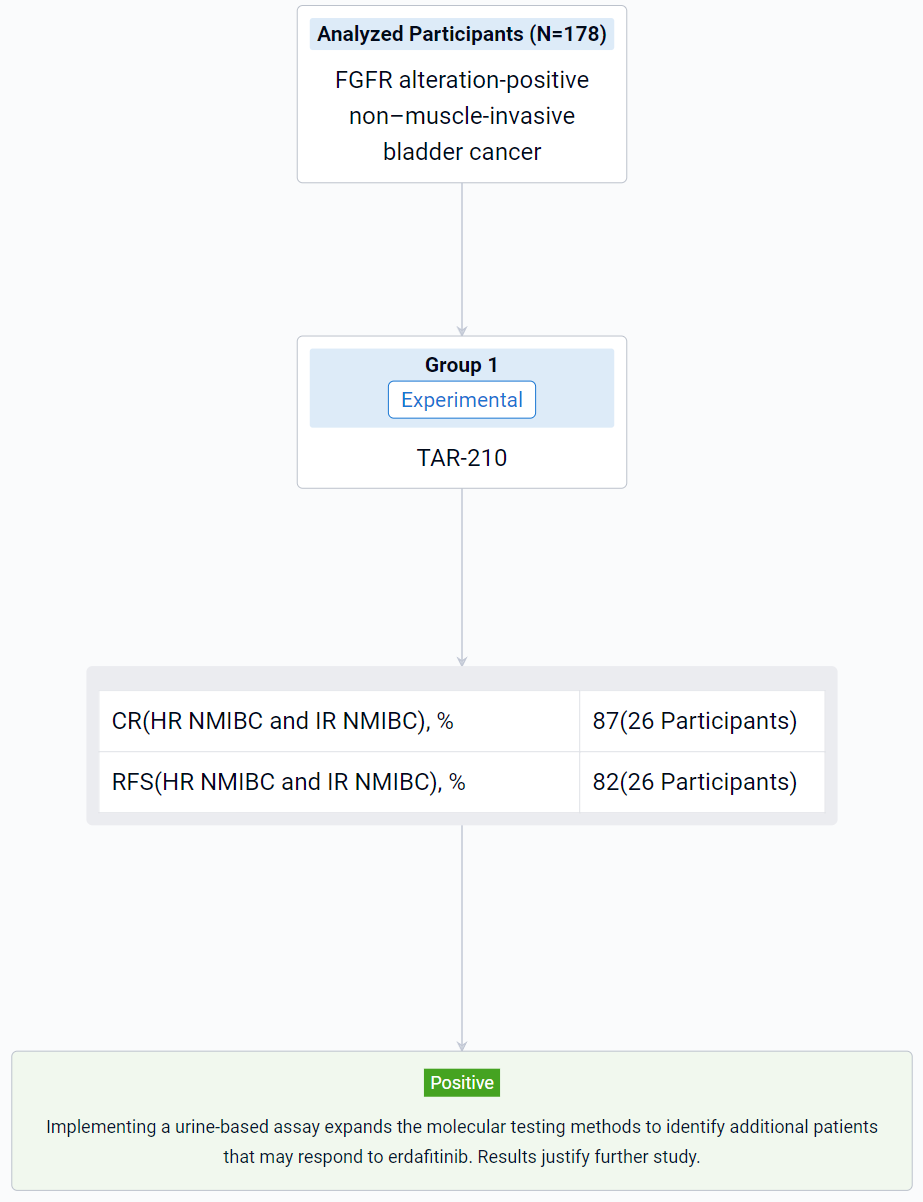
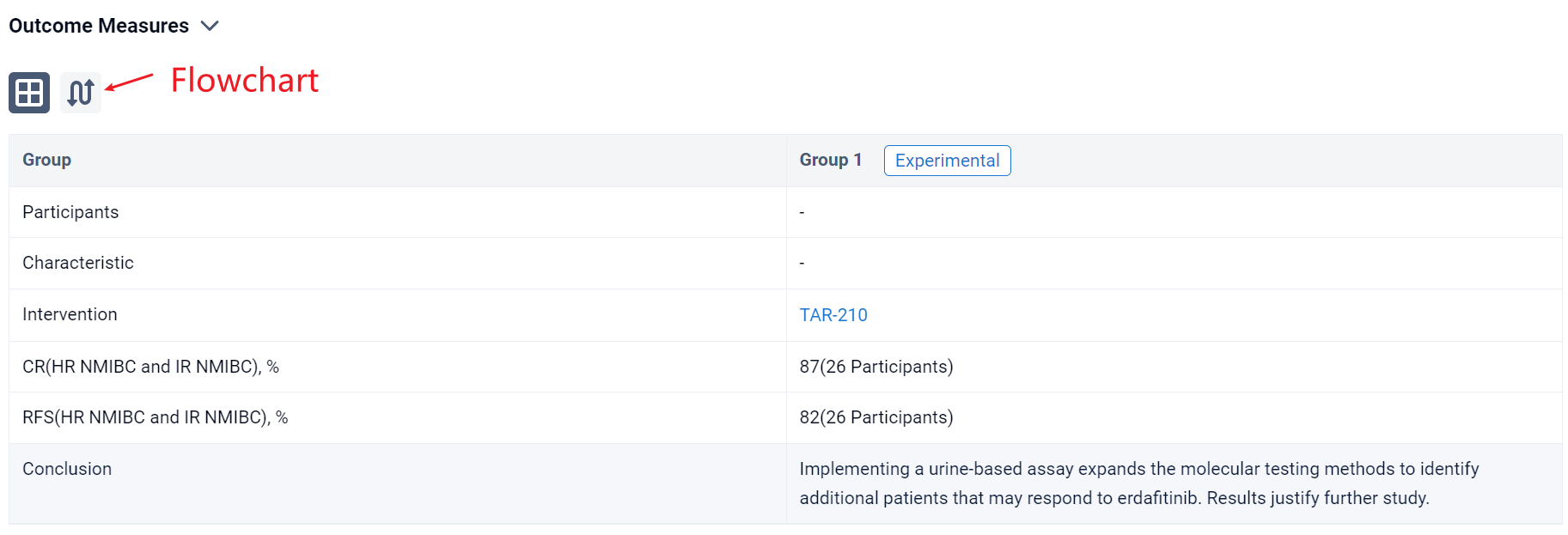
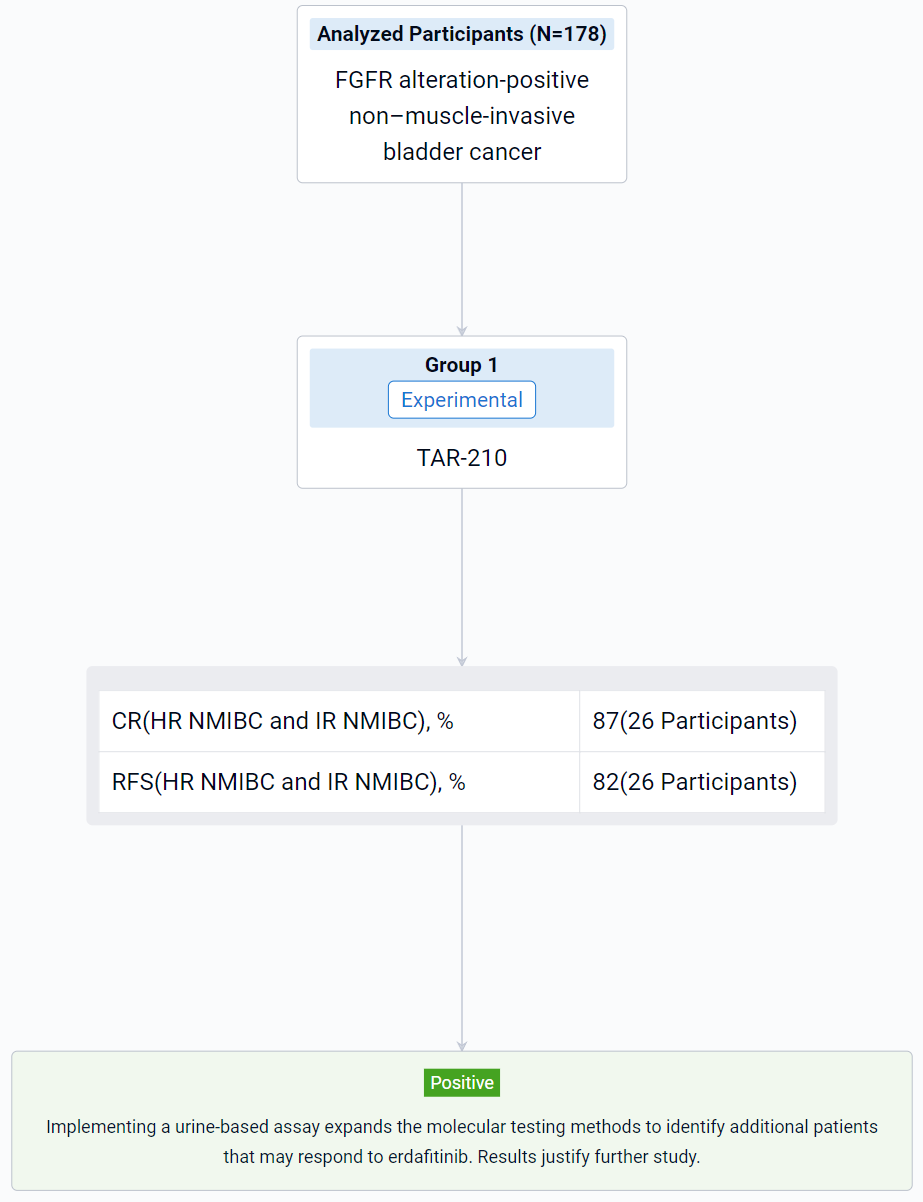
The result showed that as of Jun 20, 2023, urine assay performance was compared to the tissue test from all screened patients with NMIBC (N=178). The proportions of samples that yielded results were 60% and 58% from tissue and urine, respectively, while the FGFRalt detection rates in the subsets that yielded results were 62% and 42%, respectively. FGFR3 S249C was the most frequent alteration detected in both tissue (48%) and urine (61%). For 36% of urine samples in which FGFRalt were detected, there was no corresponding tissue result. Of the disease-evaluable patients with high-risk (HR) NMIBC (N=11) or intermediate-risk (IR) NMIBC (N=15),18.2% and 33%, respectively, were enrolled based on urine assay alone due to insufficient tissue samples. A recurrence-free (RF) rate of 82% and a complete response (CR) rate of 87% were achieved at the first disease evaluation amongst patients with HR NMIBC and IR NMIBC, respectively. All patients (HR NMIBC, N=2, and IR NMIBC, N=5) enrolled by “urine only” were RF or achieved a CR. Based on the PredicineCARE panel, comprehensive genomic assessment of urine samples from all screened patients with NMIBC was performed. The prevalence of alterations detected was similar to that described in prior studies using tissue-based testing.
It can be concluded that implementing a urine-based assay expands the molecular testing methods to identify additional patients that may respond to erdafitinib. Results justify further study.
How to Easily View the Clinical Results Using Synapse Database?
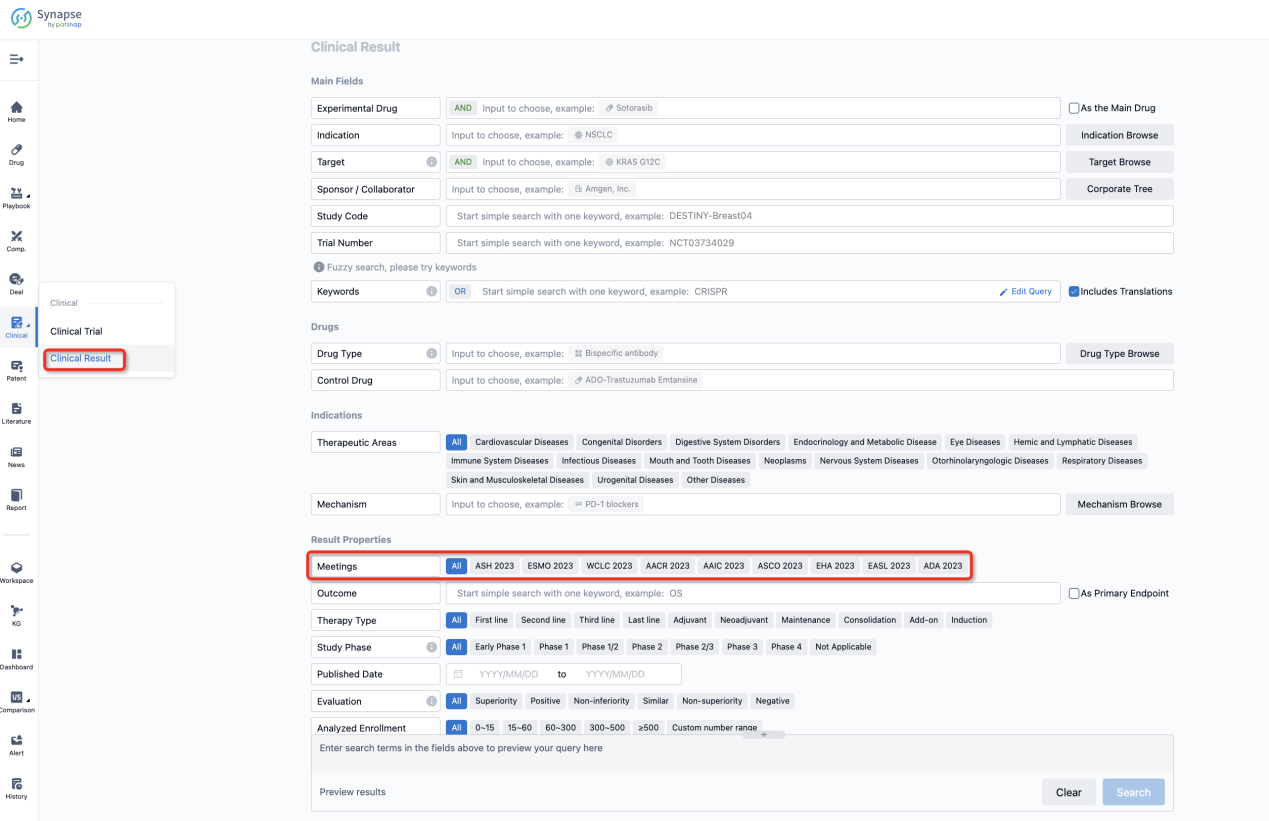
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
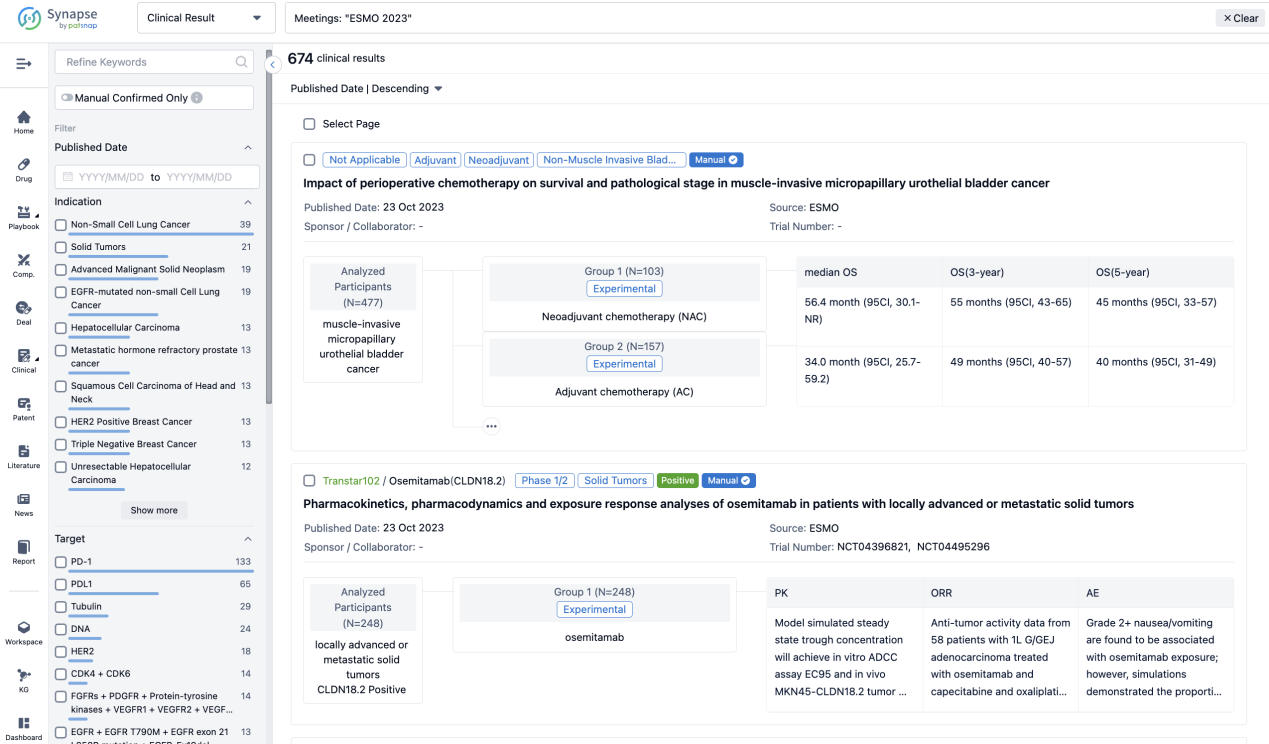
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
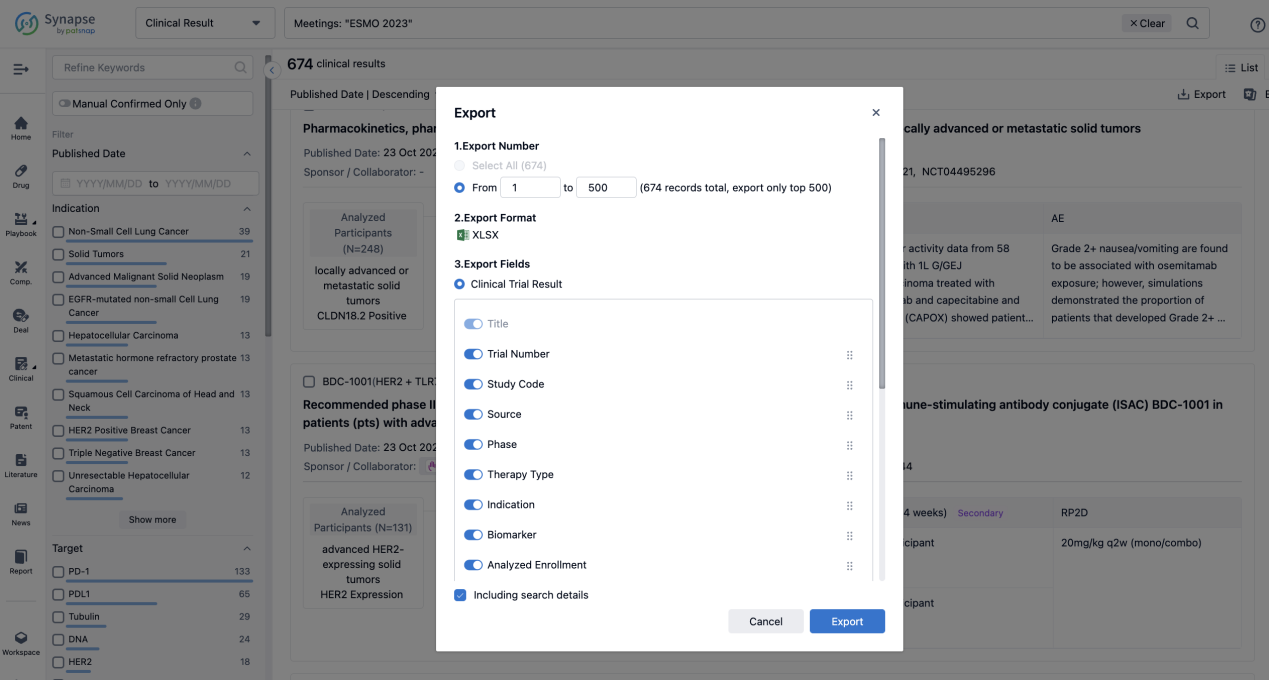
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!