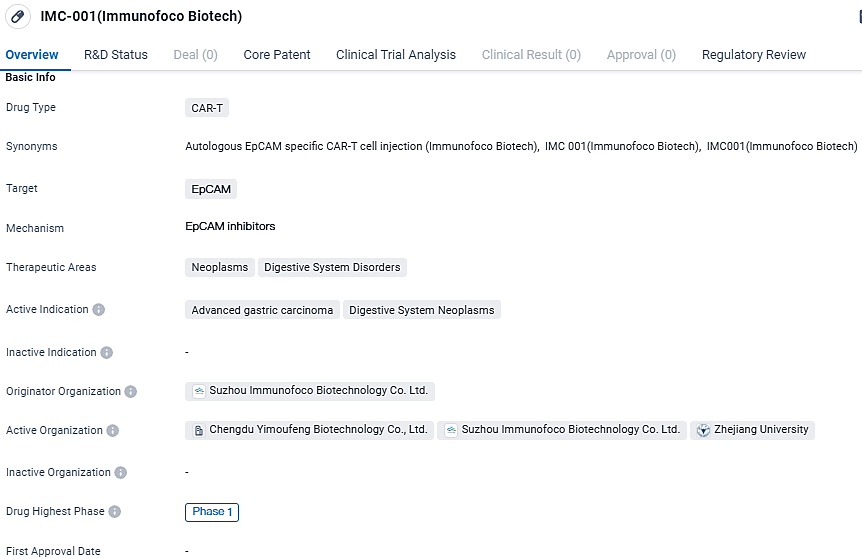
U.S. and China Approve INDs for New EpCAM CAR-T Therapy Against Solid Tumors
Immunofoco announced that subsequent to the Chinese CDE granting the go-ahead for clinical testing of their formulation IMC001, a CAR-T cellular therapy that homes in on EpCAM, they have similarly garnered clearance from the U.S. FDA for their Investigational New Drug application. This therapeutic product is designed for intravenous application in patients with EpCAM-expressing metastatic gastrointestinal neoplasms, specifically those with progressive stages of stomach cancer as well as tumors at the junction of the stomach and esophagus.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
EpCAM functions as an indicator for circulating neoplastic cells, exhibiting elevated levels in primary and metastatic areas associated with gastrointestinal malignancies, in contrast to its minimal presence in healthy tissue. This trait marks it as an advantageous target for the treatment of gastrointestinal cancer, offering an extensive array of potential applications.
IMC001, aimed at EpCAM, represents the inaugural chimeric antigen receptor T (CAR-T) therapy to adopt the novel tactic of treating solid tumors by approaching them in a manner akin to blood cancers. In the month of August 2023, this innovative therapy was acknowledged by the U.S. FDA with the Orphan Drug Designation.
The notable clinical findings of IMC001 have been highlighted at international medical symposiums, with the disclosure occurring at the European Society of Medical Oncology's yearly gathering in 2022, followed by an update at the gathering of the American Society of Clinical Oncology in 2023.
Dr. Crystal Sun, the guiding force and chief executive of Immunofoco, extended her commendations and appreciation to her team, underscoring the milestone accomplished by Immunofoco: attaining concurrent approvals to start trials on a secondary solid tumor-targeting CAR-T therapy within the United States and China.
Concentrating on EpCAM offers substantial promise for the CAR-T therapeutic approach in solid tumor management, given its abundant expression in circulating, primary, and metastatic cancer cells. EpCAM is present in roughly 90% of individuals diagnosed with gastrointestinal cancers, meeting a sizeable medical demand. With the endorsement of this Investigational New Drug (IND) by both the US and Chinese authorities, IMC001 is set to progress into additional clinical studies and cultivate global partnerships, establishing its role as an emerging treatment alternative for patients combating advanced gastrointestinal cancers on an international scale.
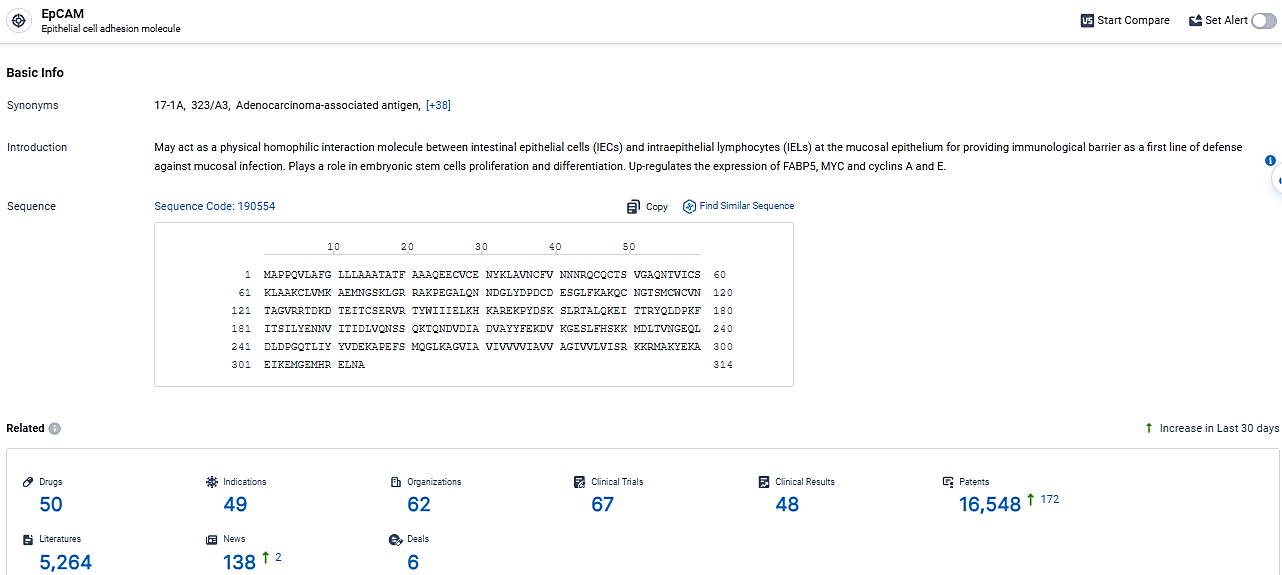
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 29, 2024, there are 50 investigational drugs for the EpCAM target, including 49 indications,62 R&D institutions involved, with related clinical trials reaching 67, and as many as 16548 patents.
IMC-001 targets EpCAM and is being investigated for its potential in treating advanced gastric carcinoma and digestive system neoplasms. Currently in Phase 1 of clinical trials, IMC-001 holds the promise of offering a new therapeutic option for patients with these conditions. As an orphan drug, it may receive additional support and incentives to facilitate its development and availability in the market. However, further research and clinical trials are necessary to determine its safety and efficacy.