Exploring the Latest OX40 inhibitors Deal by Hutchmed: A Guide to Rapidly Accessing Transaction Insights
Recently, Hutchmed and Inmagene jointly announced that in accordance with the strategic collaboration terms signed, the parties have exercised the options to license two candidate drugs, IMG-007 and IMG-004. According to the terms of the agreement, Hutchmed will grant Inmagene exclusive options for the four candidate drugs for the treatment of immune diseases. If Inmagene exercises these options, they will have the rights to further develop, manufacture, and commercialize the candidate drugs globally, while Hutchmed will retain the right of first refusal to co-commercialize in mainland China. For each candidate drug, Hutchmed is entitled to receive up to $95 million in development milestone payments, up to $135 million in commercial sales milestone payments, and up to a double-digit percentage of the net annual sales after commercialization.
About IMG-007
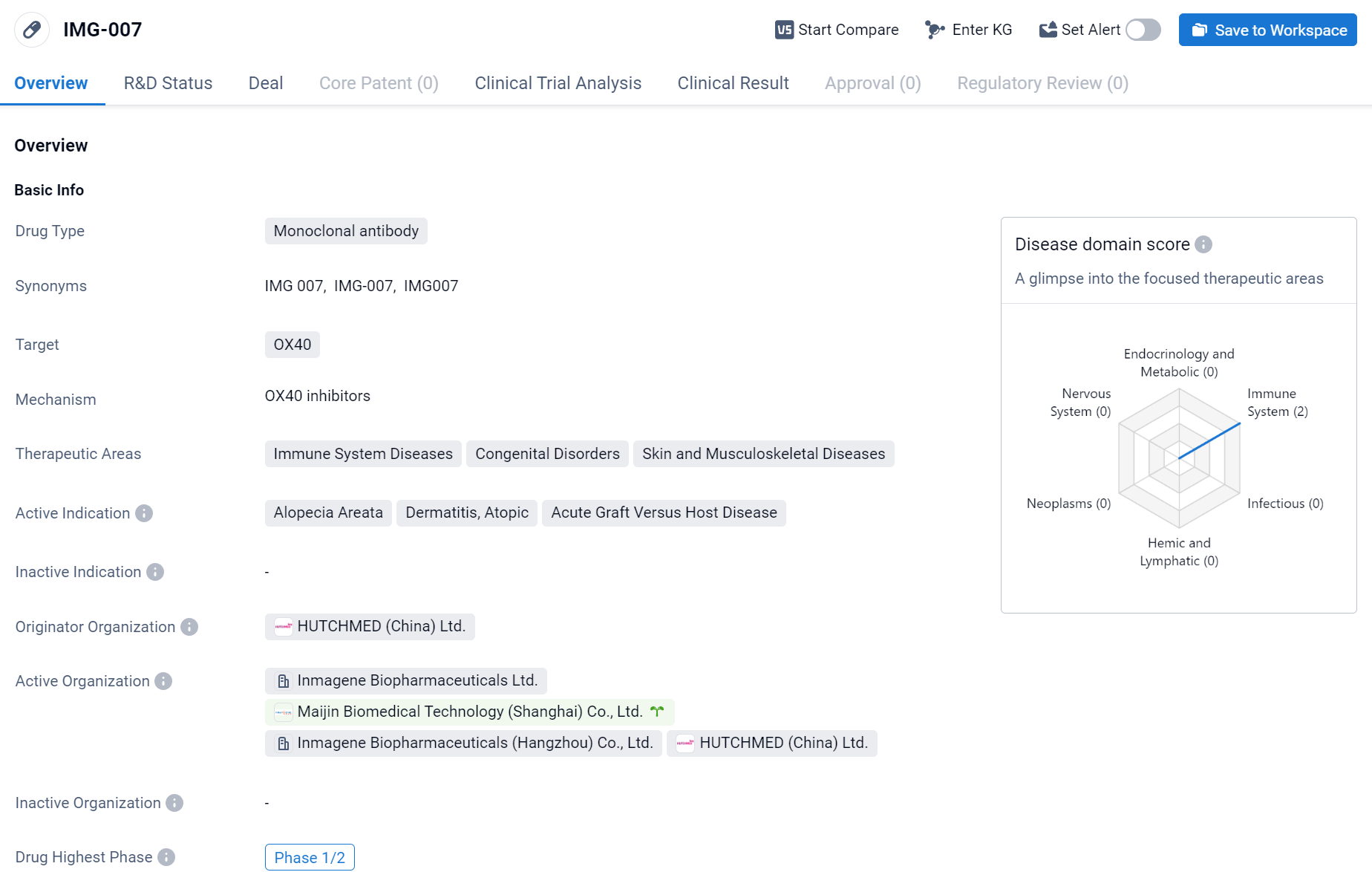
[IMG-007] is a monoclonal antibody drug developed by HUTCHMED (China) Ltd. The drug targets OX40, a protein receptor found on the surface of certain immune cells. By targeting OX40, [IMG-007] aims to modulate the immune system and potentially treat a range of immune system diseases, congenital disorders, as well as skin and musculoskeletal diseases. Currently, RBD7022 is currently in the highest phase of clinical development, Phase 1/2, globally. In China, it has received IND approval. Click the image below to directly embark on the exploration journey with the IMG-007!
In the single ascending dose study in healthy adults, IMG-007 demonstrated a half-life that exceeded the average half-life of traditional IgG antibodies. At the anticipated effective dose levels, a single administration of the drug was able to maintain antibody concentrations above the target serum levels for approximately 12-18 weeks. This suggests that IMG-007 could potentially be administered at a frequency of every 12 weeks or less, thus potentially offering patients a longer "drug holiday." IMG-007 was well tolerated at doses up to 600 mg, with no serious or severe adverse events reported, and there were no reports of fever or chills.
Currently, the company is conducting two global Phase II clinical trials. In August 2023, the first patient was dosed in a global multicenter Proof of Concept (POC) study of IMG-007 in the treatment of moderate to severe atopic dermatitis (AD) in adult patients. The study aims to evaluate the safety, pharmacokinetic characteristics, and efficacy of IMG-007 in patients with AD.
About HUTCHMED (China)
HUTCHMED (China) Ltd. is a pharmaceutical organization with a diverse portfolio of drugs targeting various therapeutic areas and molecular targets. The company has a strong focus on developing treatments for Neoplasms, with a significant number of drugs in this area. HUTCHMED's most frequently developed targets include PD-1, CD47, and IDH1 x IDH2, among others, indicating a commitment to exploring different biological pathways. The company's pipeline status shows a mix of drugs in different stages of development, with a notable presence in Phase 1 and Phase 2. Additionally, HUTCHMED has successfully obtained regulatory approval for several drugs, allowing them to be marketed and sold.
How to get the latest progress on drug deals?
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
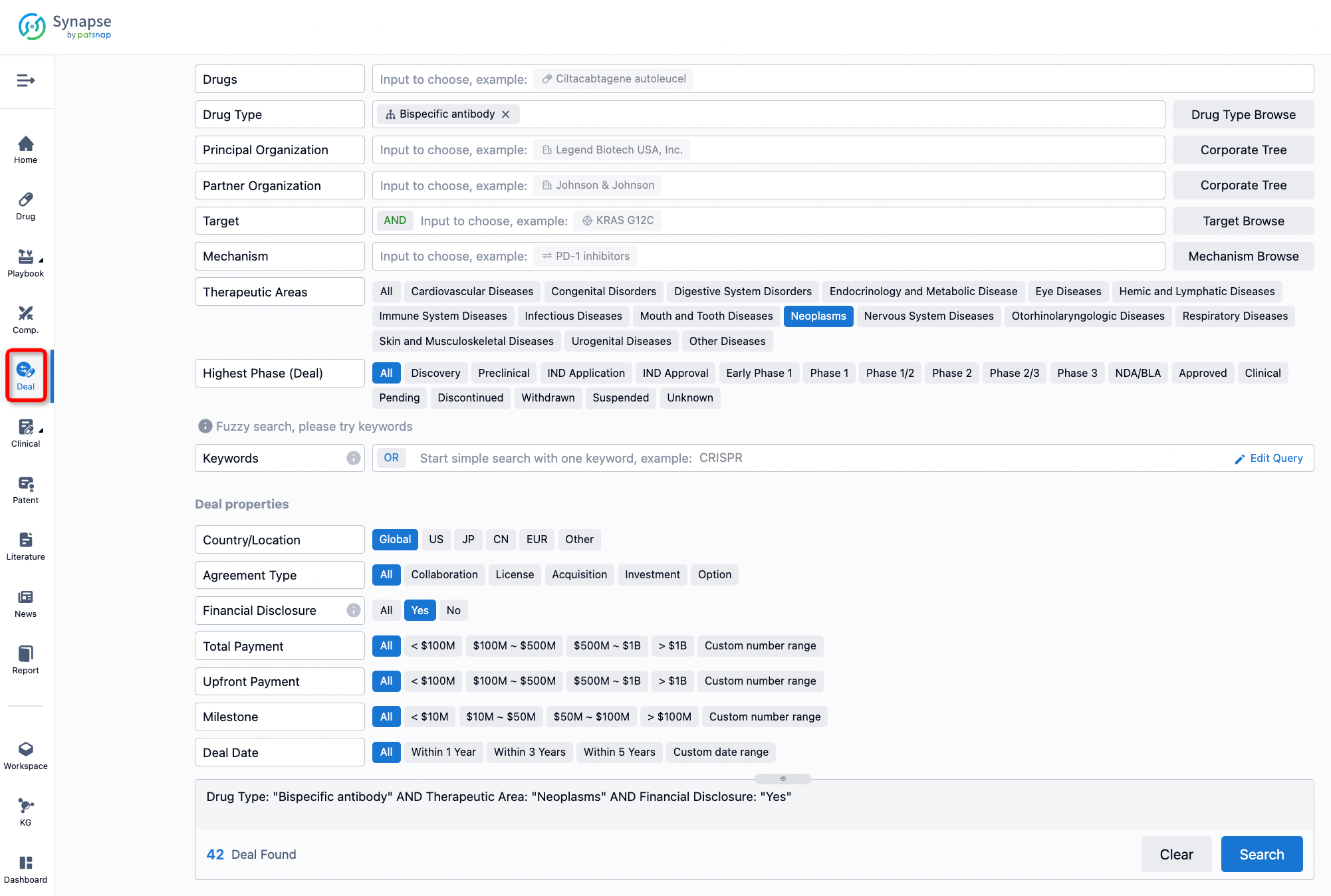
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
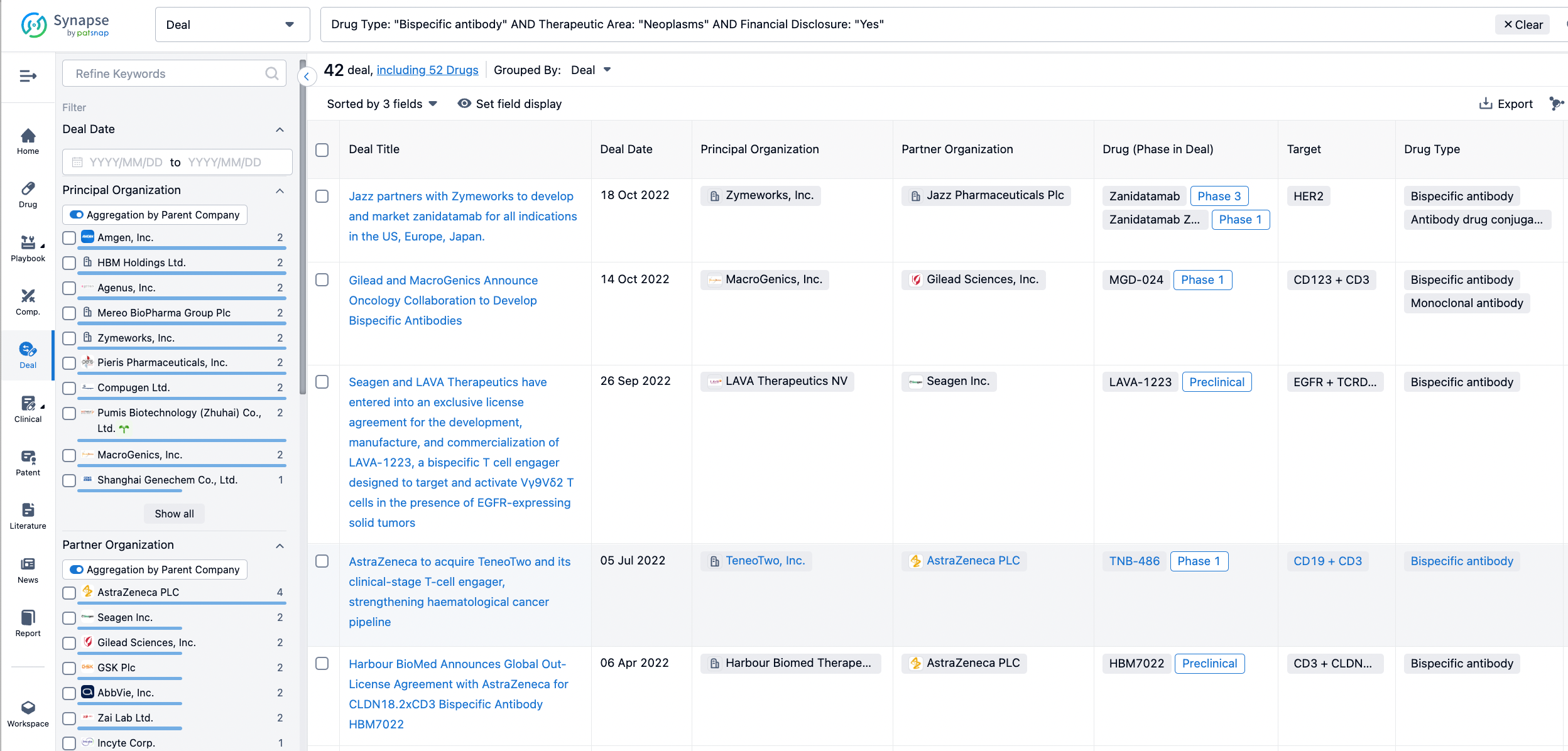
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
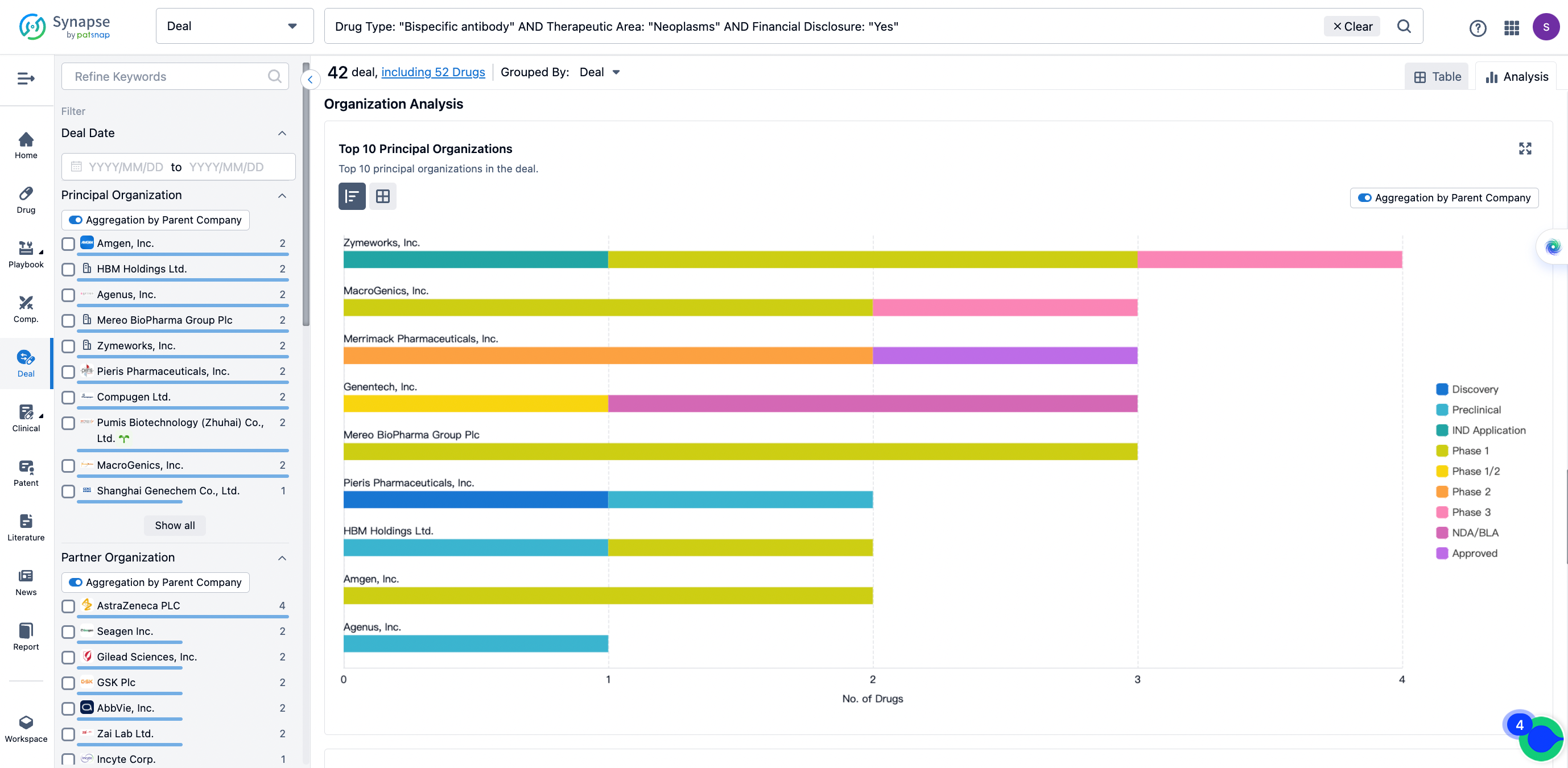
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
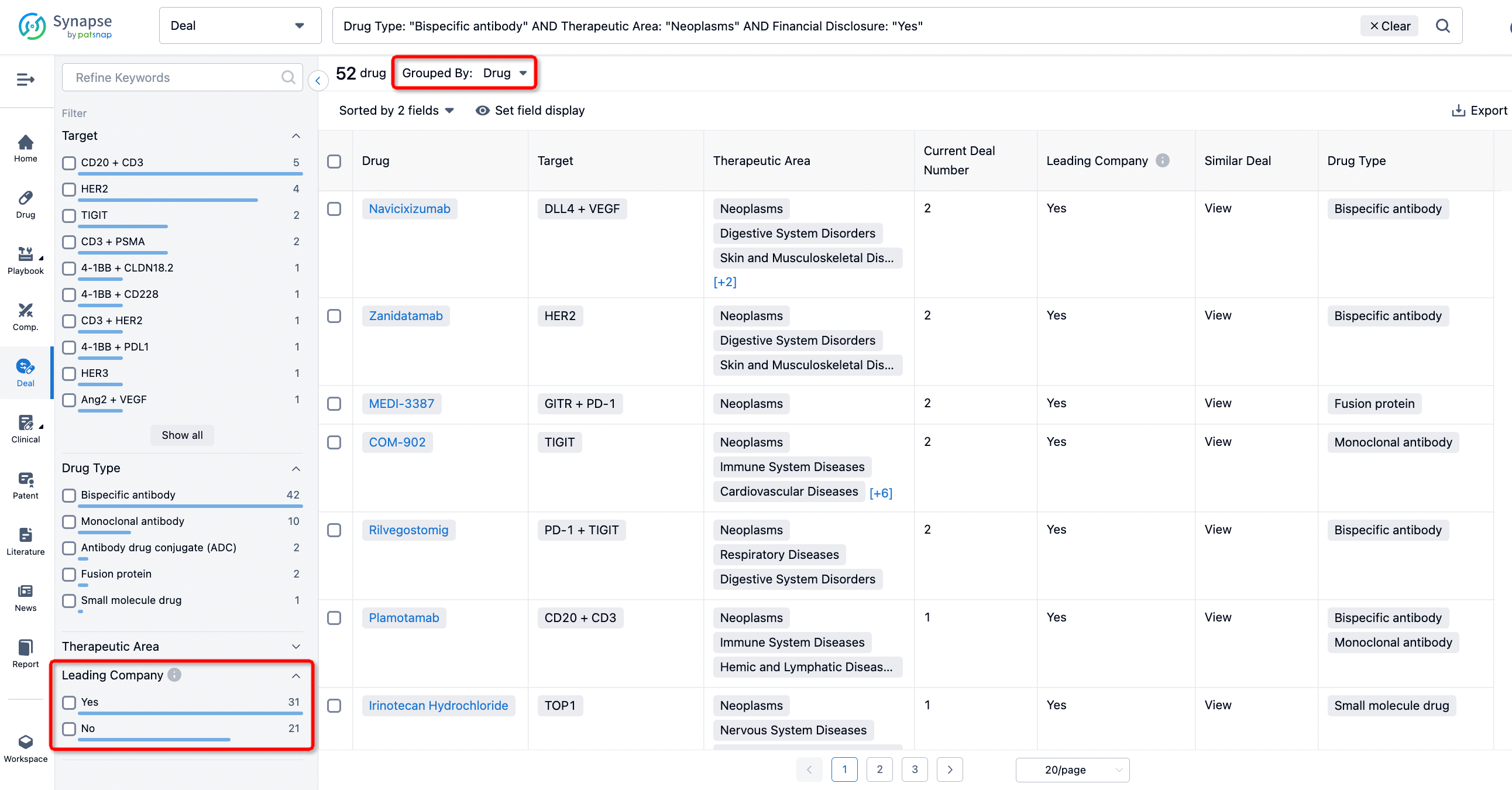
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.
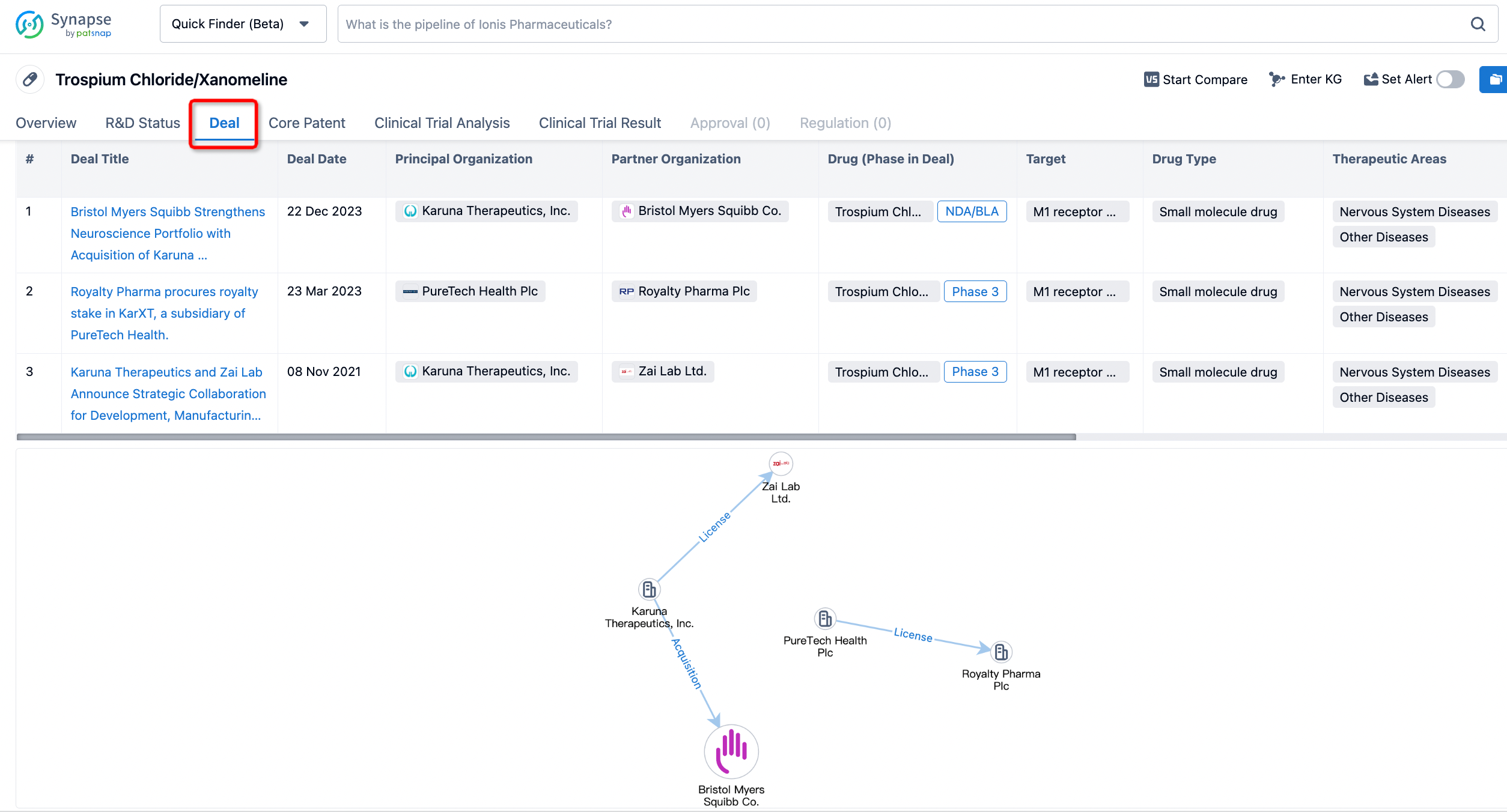
Click on the image below to embark on a brand new journey of drug discovery!