Escient Pharmaceuticals Begins First Human Study of EP262, an Oral MRGPRX2 Inhibitor for Eczema Treatment
Escient Pharmaceuticals, a company engaged in the research and development of new small molecule drugs targeting systemic neuro-immune diseases in the clinical phase, has disclosed the commencement of dosing in their Phase 2a EASE clinical trial. This trial is designed to evaluate the preliminary efficacy and safety of the compound EP262 in individuals diagnosed with atopic dermatitis.
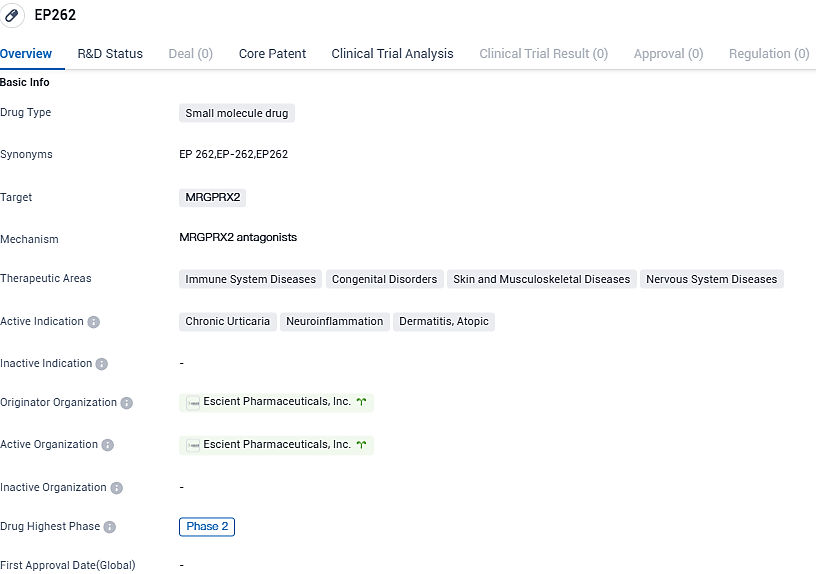
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Inhibiting the activation of MRGPRX2 and the release of compounds from mast cells, EP262 holds the promise of efficaciously managing a spectrum of diseases related to mast cell activity, initially targeting chronic urticaria and atopic dermatitis. EP262 unveils an innovative, specific strategy for addressing these conditions and potentially offers daily oral dosing without the adverse events noted in other methods.
The phase 2a clinical trial named EASE is a methodically planned, randomized, double-masked, and placebo-matched study purposed to assess the safety, tolerability, and pharmacodynamic responses of EP262 in around 30 subjects suffering from moderate to grievous AD. Study participants will be allocated, by a 2:1 division, to receive either EP262 at a 150 mg dosage or a placebo, taken orally each day for a six-week span, which will be succeeded by a period without treatment for evaluation.
Data from studies before clinical trials, utilizing a sophisticated mouse model that emulates many major aspects of human AD, indicated that oral administration of EP262 reduced symptoms and signs consistent with AD skin lesions and diminished type 2 inflammatory markers. Intriguingly, the therapeutic performance seen with EP262 mirrored those observed with anti-IL4 treatments in the same animal model. Select findings from this research were shared at the yearly conference of the American Academy of Dermatology in March 2023, as showcased in a press announcement released on March 16, 2023.
“As we commence our EASE study to provide clinical validation of concept, we are eager to determine if the encouraging results from EP262 preclinical trials can be replicated in patients afflicted with atopic dermatitis,” declared Christian Weyer, M.D., M.A.S, the Chief Medical Officer at Escient. “Given positive outcomes, EP262 may emerge as a distinctive, daily oral therapeutic choice.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
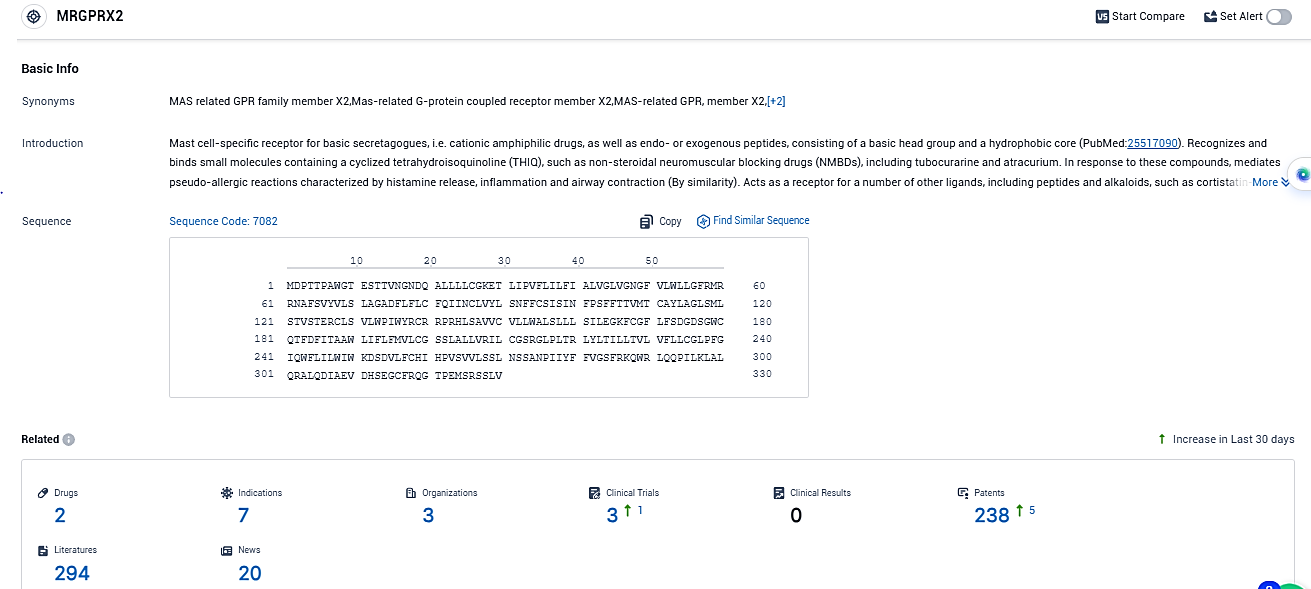
According to the data provided by the Synapse Database, As of December 5, 2023, there are 2 investigational drugs for the MRGPRX2 transcription factors target, including 7 indications, 3 R&D institutions involved, with related clinical trials reaching 3, and as many as 238 patents.
EP262 is a potent, highly selective once-daily small molecule antagonist of MRGPRX2, a receptor expressed on mast cells that is activated by numerous ligands, including many peptides released from sensory neurons as well as other cell types. Escient’s preclinical data demonstrates that, by blocking activation of MRGPRX2, EP262 has the potential to effectively treat a broad range of mast cell-mediated diseases, with an initial focus on chronic urticarias and atopic dermatitis.