What are HDAC inhibitors and how do you quickly get the latest development progress?
HDACs, or histone deacetylases, also known as histone deacetylase, is a crucial enzyme in the process of histone modification. HDAC can remove the acetyl group on the lysine residues of histones, changing the charge to make the chromosomal structure more compact, thereby inhibiting gene transcription.
Based on the homology of yeast proteins, the 18 types of HDAC currently found in the human body can be divided into four classes (Class I, II, III, and IV). Class I (HDAC1,2,3,8) are HDACs specifically expressed in the cell nucleus, Class II (HDAC4,5,6,7,9, and 10) can move between the cell nucleus and cytoplasm, Class IV (HDAC11) is similar to Class I and II but has higher tissue specificity. Class III (SIRT1,2,3,4,5,6, and 7) Sirtuins are NAD+ dependent proteins, non-metal dependent, and do not have a zinc ion catalytic site. Correspondingly, the mainstream HDAC inhibitors also have four major types. The mainstream HDAC inhibitors target Classes I, II, and IV, according to the chemical structure of the functional group that binds with Zn2+, mainly including hydroxamic acid, cyclic tetrapeptide, short-chain fatty acids, and benzamide. The first three types of compounds inhibit all HDAC subtypes of Class I and II and belong to non-selective HDAC inhibitors. Benzamides mainly inhibit HDAC1, HDAC2, HDAC3 in Class I and some Class II HDAC, they are selective and can target specific sites. In gastric cancer and rectal cancer, the expression of the HDAC family is significantly higher than normal levels.
Currently, HDACi has made significant progress in the treatment of peripheral T-cell lymphoma (PTCL). The FDA has approved HDACi belinostat, vorinostat, and romidepsin for the treatment of relapsed/refractory PTCL (rr-PTCL). After HDACi and chemotherapy are used together, their efficacy is outstanding. The NMPA has approved chidamide for the treatment of relapsed/refractory PTCL. Therefore, many drug developers believe that HDAC inhibitors have great research and development value in cancer treatment.
The mechanism of action of HDAC inhibitors
HDAC inhibitors are a type of drugs that inhibit the activity of histone deacetylases (HDACs). HDACs are enzymes that remove acetyl groups from histone proteins, which are involved in the packaging of DNA in the nucleus. By inhibiting HDACs, HDAC inhibitors increase the acetylation of histones, leading to changes in gene expression.
From a biomedical perspective, HDAC inhibitors have been studied for their potential therapeutic applications in various diseases, including cancer. Altered gene expression is a hallmark of cancer, and HDAC inhibitors can modulate gene expression patterns by affecting the acetylation status of histones. This can result in the reactivation of tumor suppressor genes, inhibition of oncogenes, and induction of cell cycle arrest, apoptosis, and differentiation in cancer cells.
In addition to cancer, HDAC inhibitors have also shown promise in the treatment of other conditions such as neurodegenerative disorders, inflammatory diseases, and cardiovascular diseases. They have the potential to regulate gene expression and modify cellular processes involved in these diseases.
It's worth noting that HDAC inhibitors can have side effects, including gastrointestinal disturbances, fatigue, and hematological abnormalities. Therefore, their use as therapeutic agents requires careful consideration and monitoring.
Overall, HDAC inhibitors are a class of drugs that target histone deacetylases, modulating gene expression and potentially offering therapeutic benefits in various diseases.
Catalog of HDAC Inhibitors
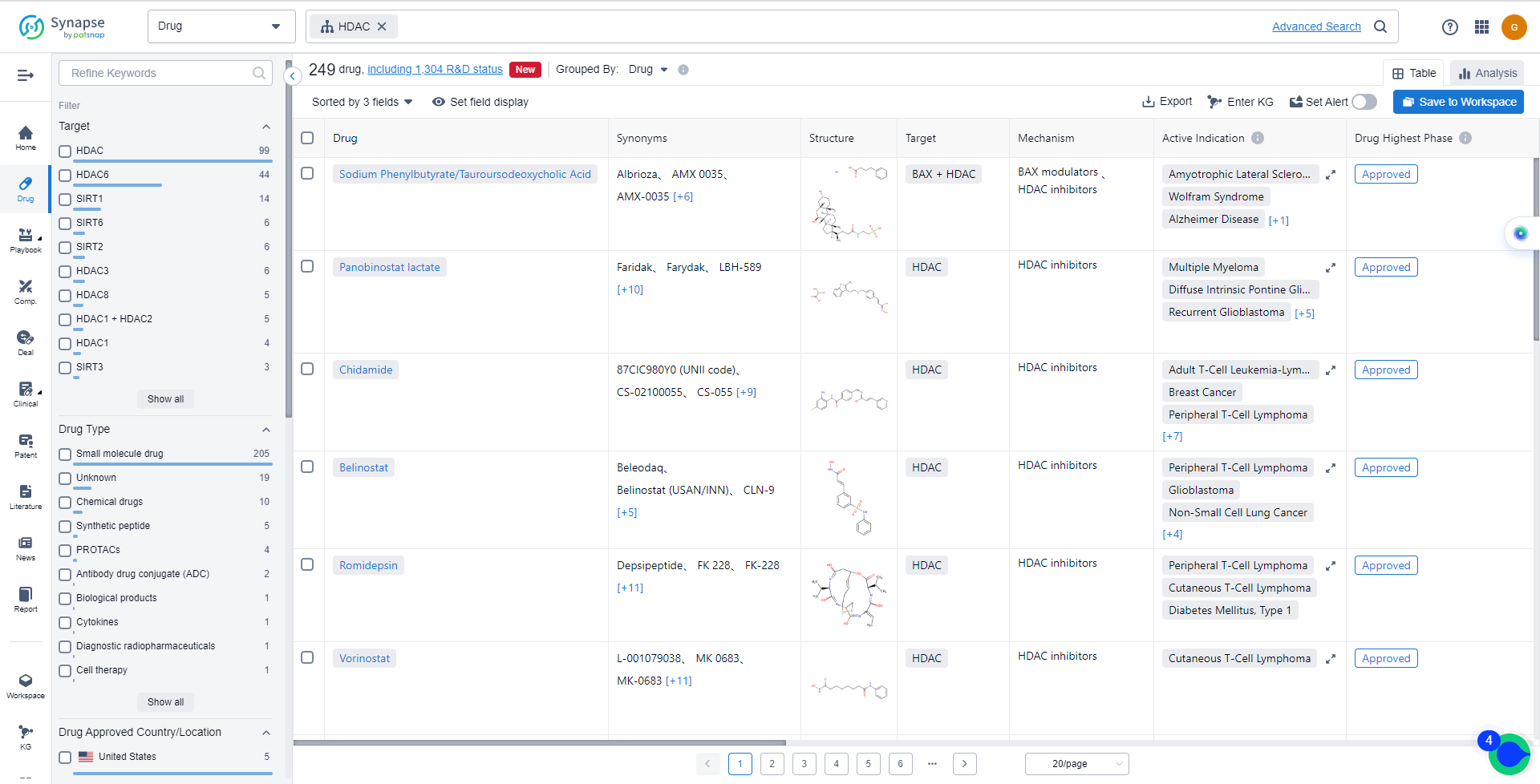
The currently marketed HDAC inhibitors include:
- Sodium Phenylbutyrate/Tauroursodeoxycholic Acid
- Panobinostat lactate
- Chidamide
- Belinostat
- Romidepsin
- Vorinostat
- Sodium Phenylbutyrate
- BEBT-908
- Entinostat
- Givinostat Hydrochloride
For more information, please click on the image below.
What is the purpose of using HDAC inhibitors?
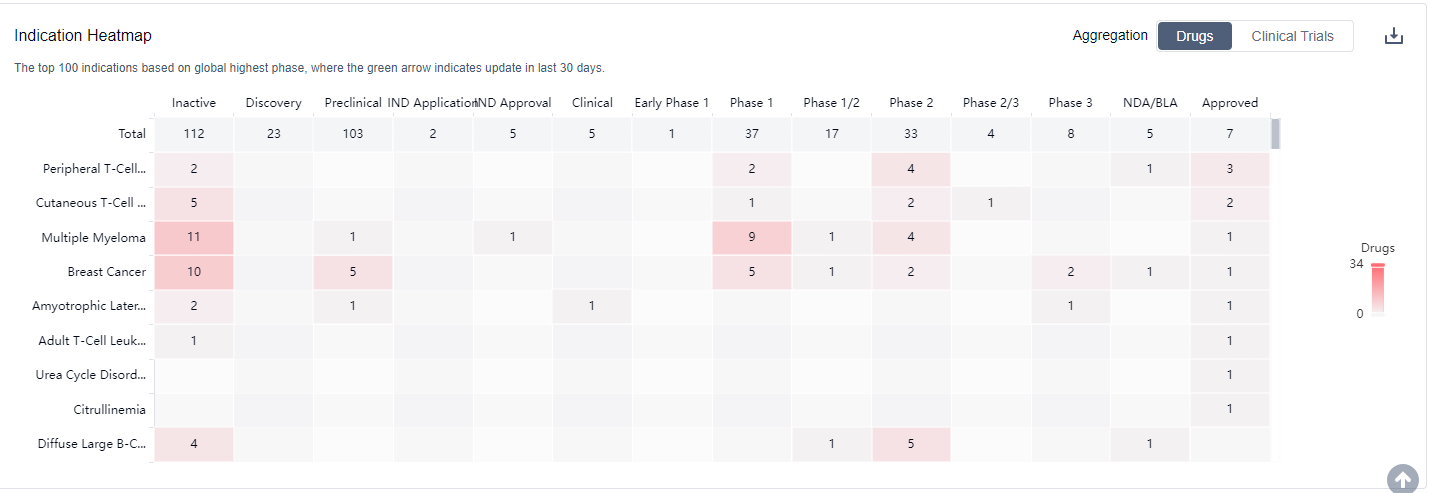
As a mature anti-cancer target, HDAC inhibitors currently have indications mainly focused on blood diseases, and most products are undergoing clinical trials for new indications, such as breast cancer, non-small cell lung cancer, and pancreatic cancer. For more information, please click on the image below to log in and search.
How can I get the most recent advancements in HDAC inhibitors?
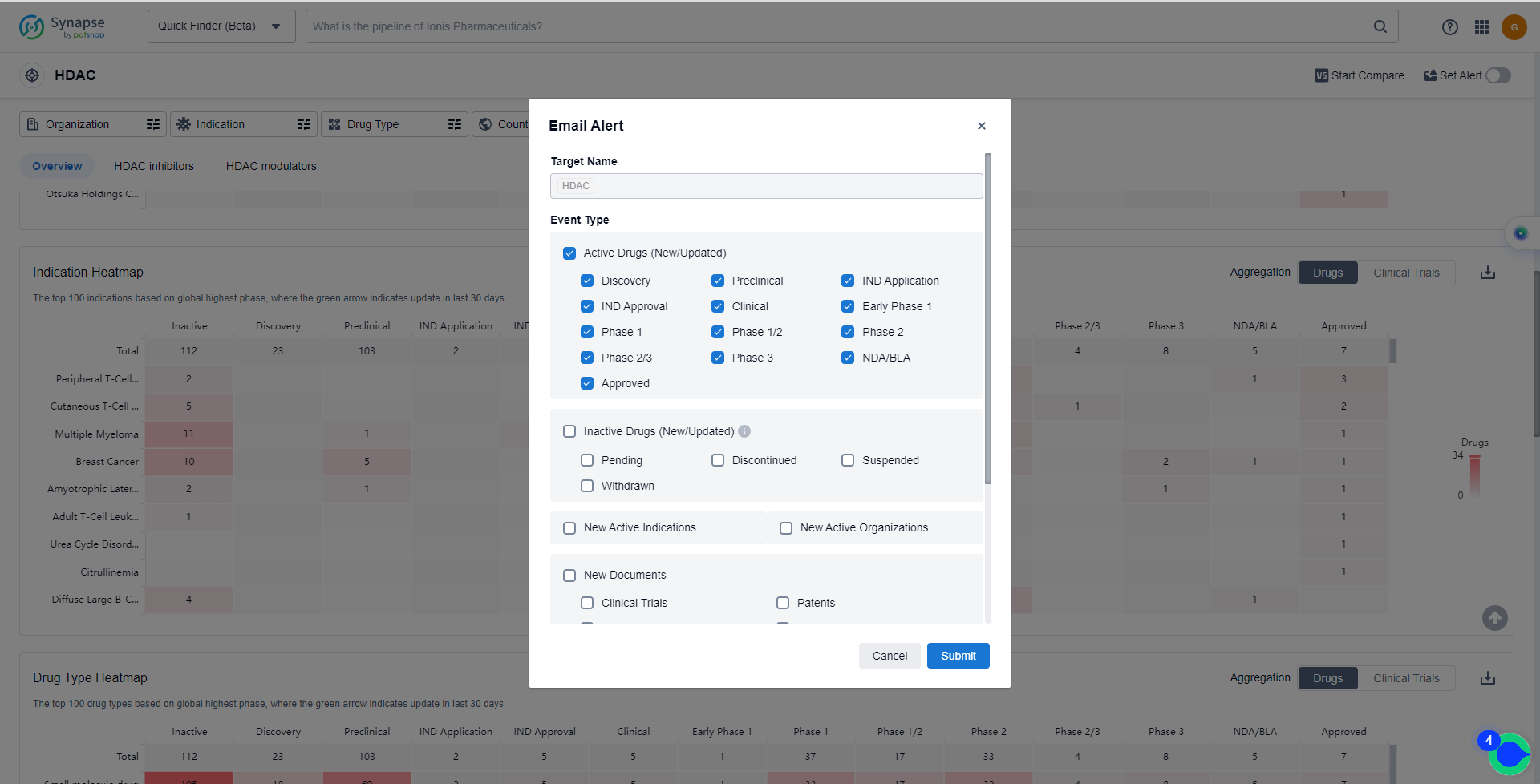
In the Synapse database, you can keep abreast of the latest research and development advances of HDAC inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!