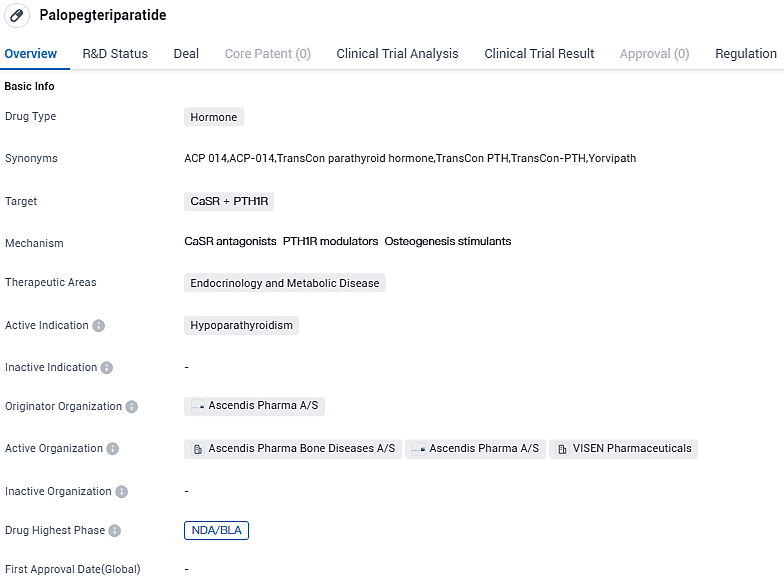
European Commission approves Ascendis Pharma's YORVIPATH® (palopegteriparatide) for treating chronic Hypoparathyroidism in adults
Ascendis Pharma A/S has disclosed that the EC has granted approval for the selling and distribution of YORVIPATH® (palopegteriparatide), which is used as a replacement therapy for adult patients suffering from long-term hypoparathyroidism. YORVIPATH is a prodrug of the parathyroid hormone and is to be taken once a day. Ascendis is set to launch YORVIPATH in the EU for the first time in January 2024, starting in Germany.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"People living with chronic hypoparathyroidism often experience a range of health and quality of life challenges," explained Prof. Lorenz C. Hofbauer, Professor of Medicine, Geriatrics, and Endocrinology at Technical University of Dresden. “It's crucial that we develop new means of treating the root cause of this disease, rather than just its symptoms. These currently include oral calcium and active vitamin D, but these conventional therapies have their limitations and potential risks."
Following a positive assessment by the European Medicines Agency's Committee for Medicinal Products for Human Use on September 14, 2023, YORVIPATH has now been approved by the European Commission.
Jan Mikkelsen, CEO and President of Ascendis Pharma, discussed their journey in the development of YORVIPATH. “We centered our focus on fulfilling the needs of patients, basing our actions on scientific findings, which has allowed us to receive EU marketing authorization for YORVIPATH – a second approved TransCon product – in merely eight years, with plans to release it in Germany by January. Aware of the urgent requirement expressed by numerous patients and healthcare providers for innovative treatment options, we will strive to make YORVIPATH accessible on a broader scale.”
YORVIPATH (also known as TransCon PTH), a parathyroid hormone replacement therapy, will be marketed in the EU for the management of adults suffering from chronic hypoparathyroidism. Physicians or healthcare professionals experienced in diagnosing and treating hypoparathyroidism should supervise its initiation and ongoing use. TransCon PTH is under development for adults with hypoparathyroidism in the US, Japan, and elsewhere.
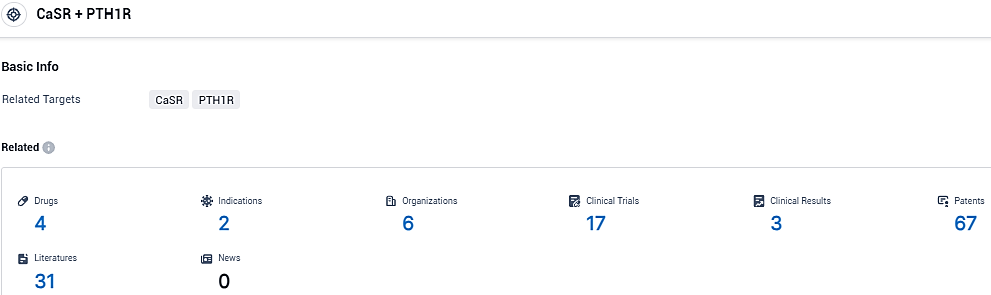
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 28, 2023, there are 4 investigational drugs for the CaSR and PTH1R target, including 2 indications, 6 R&D institutions involved, with related clinical trials reaching 17, and as many as 67 patents.
Palopegteriparatide shows promise as a hormone drug targeting CaSR and PTH1R for the treatment of hypoparathyroidism. The fact that it has reached the NDA/BLA stage globally and Phase 3 in China suggests that it has undergone rigorous testing and has demonstrated positive results in clinical trials. The priority review and orphan drug designations further highlight the potential value of Palopegteriparatide in addressing the specific medical needs of patients with hypoparathyroidism.