Exploring Iptacopan's R&D successes and its clinical results at the 2023 ASH
On 11 Dec 2023, the final phase III APPLY-PNH trial data after a 24-wk extension period in which all pts received iptacopan monotherapy will be reported in 2023 ASH.
Iptacopan's R&D Progress
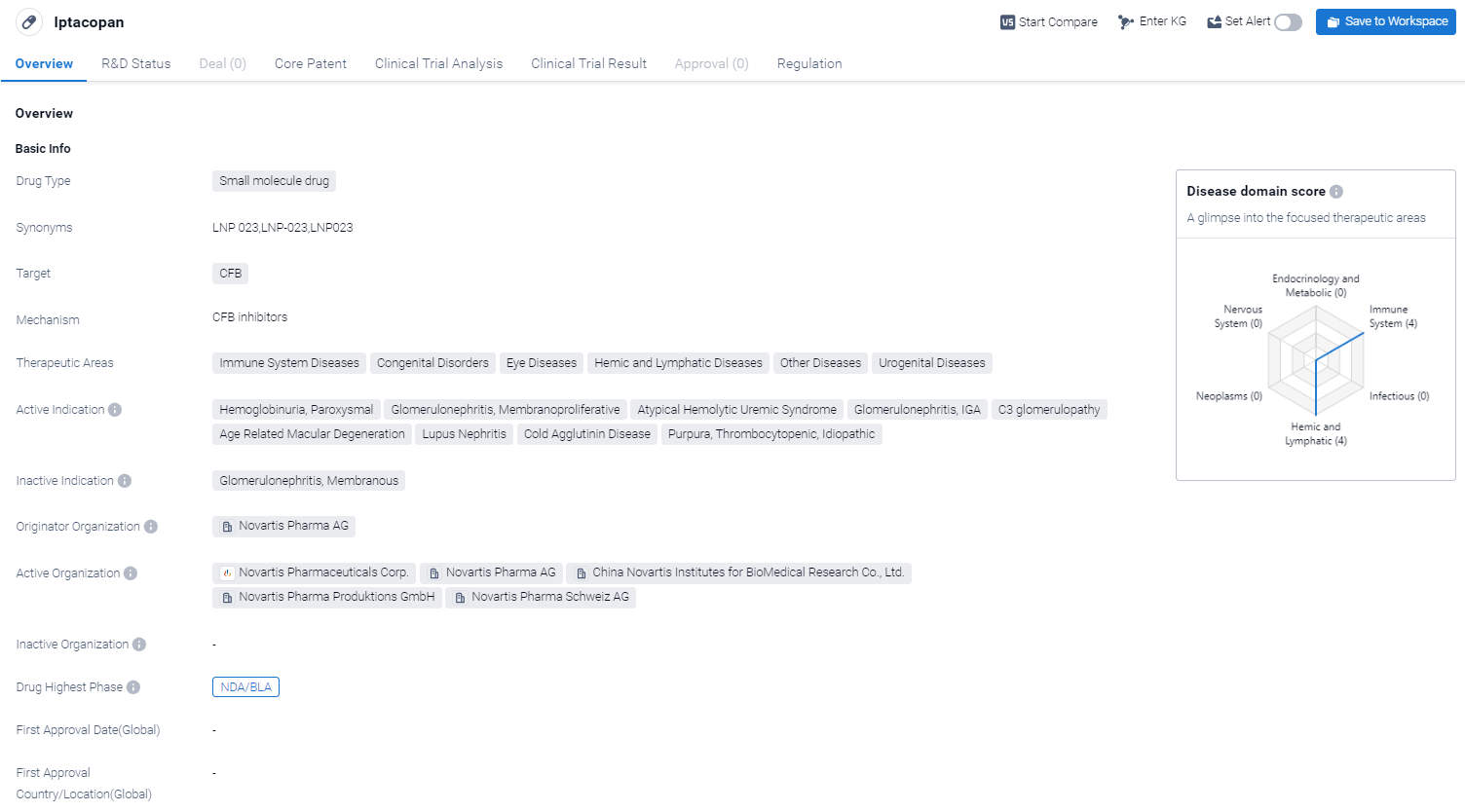
Iptacopan is a small molecule drug that is being developed by Novartis Pharma AG. It targets the CFB protein. In terms of therapeutic areas, Iptacopan has shown promise in treating immune system diseases, congenital disorders, eye diseases, hemic and lymphatic diseases, as well as other diseases and urogenital diseases.
According to the Patsnap Synapse, Iptacopan is currently in the highest phase of development, known as NDA/BLA, globally. And the clinical trial areas for Iptacopan are primarily in the United States, China and United Kingdom.The key indication is Immune System Diseases. 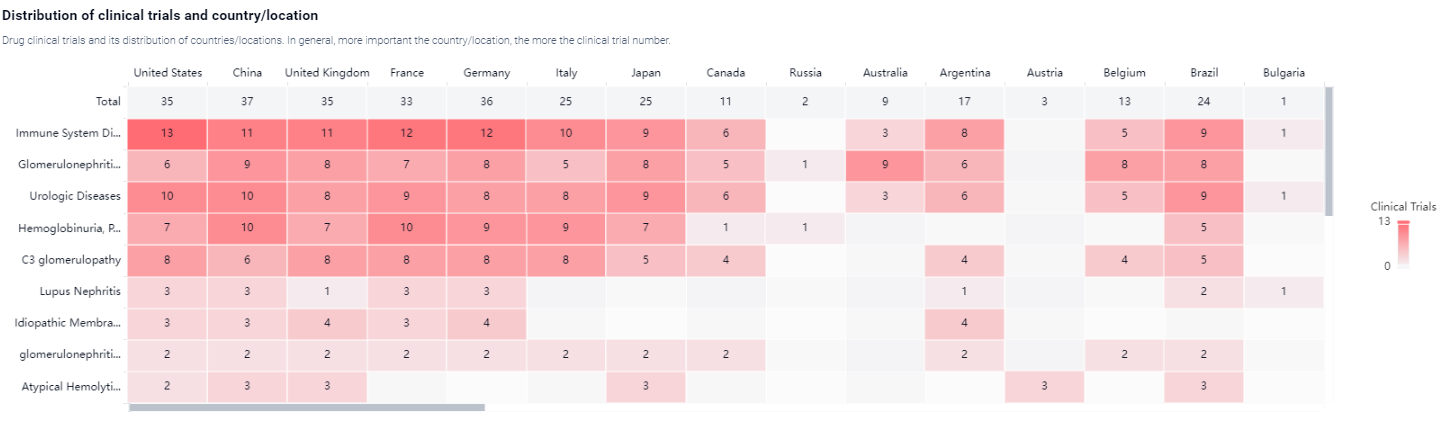
Detailed Clinical Result of Iptacopan
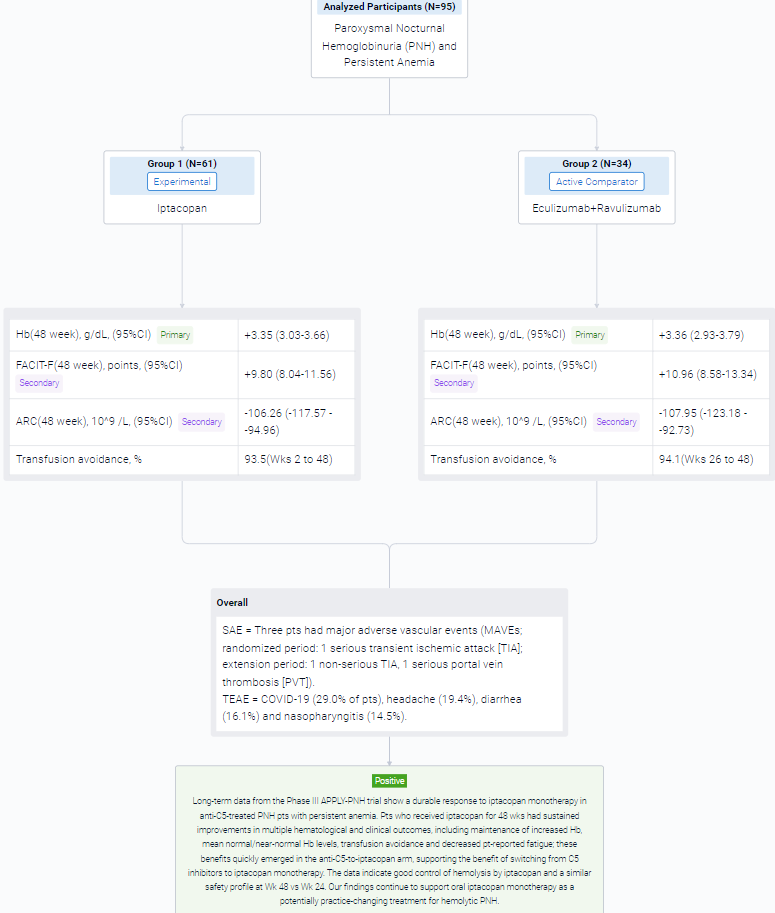
This randomized, parallel assignment, open-labeled clinical trial (NCT04558918) was aimed to investigate the safety and efficacy of Iptacopan in anti-C5-treated patients (pts) with paroxysmal nocturnal hemoglobinuria (PNH) and persistent anemia.
In this study, adult PNH pts (mean Hb <10 g/dL, receiving anti-C5 therapy for ≥6 months) were randomized to receive iptacopan 200 mg twice daily or continue their anti-C5 regimen for 24 wks. Pts could then opt to enter an extension period; pts in the iptacopan arm received iptacopan for another 24 wks and pts who had been receiving anti-C5 switched to iptacopan monotherapy.
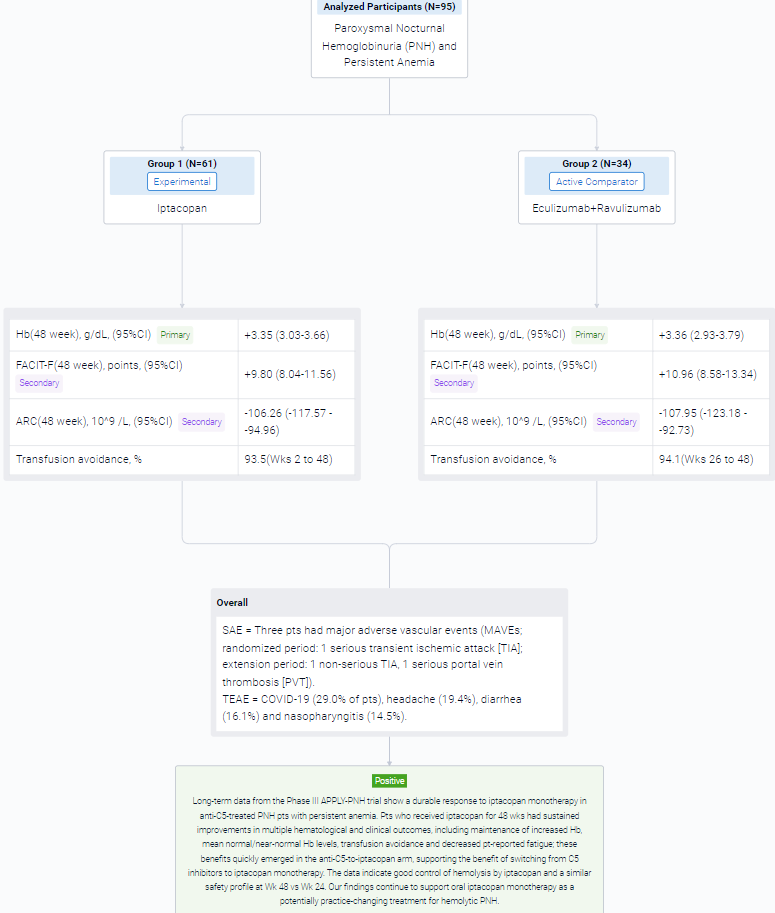
The result showed that in the extension period, 95 pts received iptacopan: 61/62 in the iptacopan arm (1 discontinued iptacopan in the randomized period because of pregnancy) and 34/35 in the anti-C5-to-iptacopan arm (1 did not enter the extension period [investigator’s decision]). In the iptacopan arm, the improvements at 24 wks were sustained at 48 wks, with maintenance of increased Hb, normal/near-normal mean Hb levels (Figure), improved Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores, decreased absolute reticulocyte counts (ARCs) and transfusion avoidance (Table). Pts who switched from anti-C5 to iptacopan had rapid changes in Hb, FACIT-F and ARC, achieving comparable improvements to the iptacopan arm. Mean Hb levels at Wk 48 were 12.2 and 12.1 g/dL in the iptacopan and anti-C5-to-iptacopan arms, respectively (standard deviations 1.6 and 1.4). At Wk 48, the adjusted mean change from baseline in the iptacopan arm was +3.35 g/dL for Hb, +9.80 FACIT-F points and −106.26 × 109/L for ARC. In the anti-C5-to-iptacopan arm, the adjusted mean change from baseline at Wk 48 was +3.36 g/dL for Hb, +10.96 FACIT-F points and −107.95 × 109/L for ARC (adjusted mean difference in change from baseline at Wk 48 vs Wk 24: +3.02 g/dL, +10.79 points and −102.29 × 109/L, respectively). Transfusion avoidance was achieved by 93.5% of pts in the iptacopan arm (Wks 2 to 48) and 94.1% in the anti-C5-to-iptacopan arm (Wks 26 to 48). Mean lactate dehydrogenase levels were generally maintained <1.5 × upper limit of normal in both arms.
In the trial, 6/62 pts in the iptacopan arm had clinical breakthrough hemolysis (BTH). One pt in the anti-C5-to-iptacopan arm had clinical BTH after switching to iptacopan. BTH resolved without changing iptacopan dosing. Three pts had major adverse vascular events (MAVEs; randomized period: 1 serious transient ischemic attack [TIA]; extension period: 1 non-serious TIA, 1 serious portal vein thrombosis [PVT]). The pt with PVT had a history of PVT and discontinued heparin prior to the MAVE. All MAVEs were considered unrelated to iptacopan and resolved without changing iptacopan dosing. After 48 wks in the iptacopan arm, the most frequently reported treatment-emergent adverse events (TEAEs) were COVID-19 (29.0% of pts), headache (19.4%), diarrhea (16.1%) and nasopharyngitis (14.5%). There were no deaths, no serious hemolysis TEAEs on iptacopan, no serious infections caused by N. meningitidis, S. pneumoniae or H. influenzae and no pts discontinued treatment because of TEAEs.
It can be concluded that long-term data from the Phase III APPLY-PNH trial show a durable response to iptacopan monotherapy in anti-C5-treated PNH pts with persistent anemia. Pts who received iptacopan for 48 wks had sustained improvements in multiple hematological and clinical outcomes, including maintenance of increased Hb, mean normal/near-normal Hb levels, transfusion avoidance and decreased pt-reported fatigue; these benefits quickly emerged in the anti-C5-to-iptacopan arm, supporting the benefit of switching from C5 inhibitors to iptacopan monotherapy. The data indicate good control of hemolysis by iptacopan and a similar safety profile at Wk 48 vs Wk 24.
How to Easily View the Clinical Results Using Synapse Database?
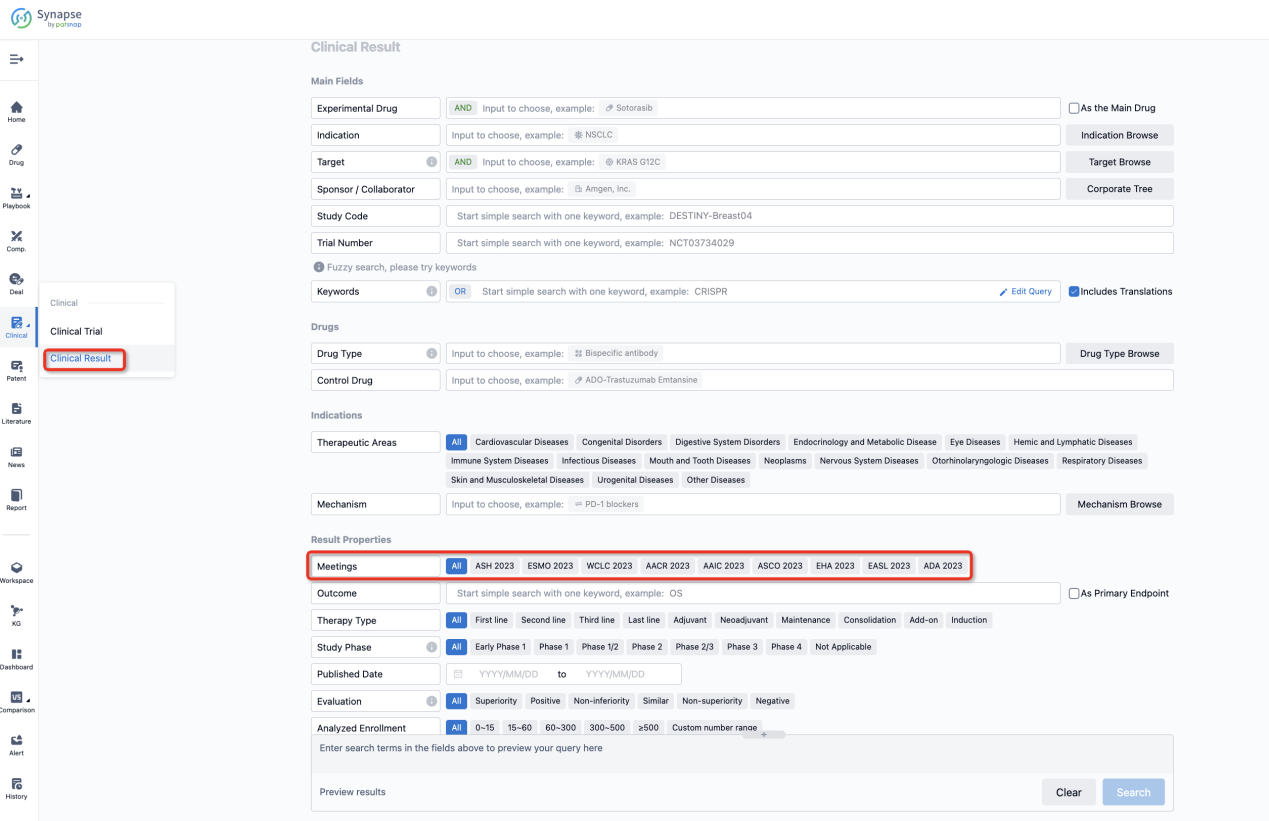
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
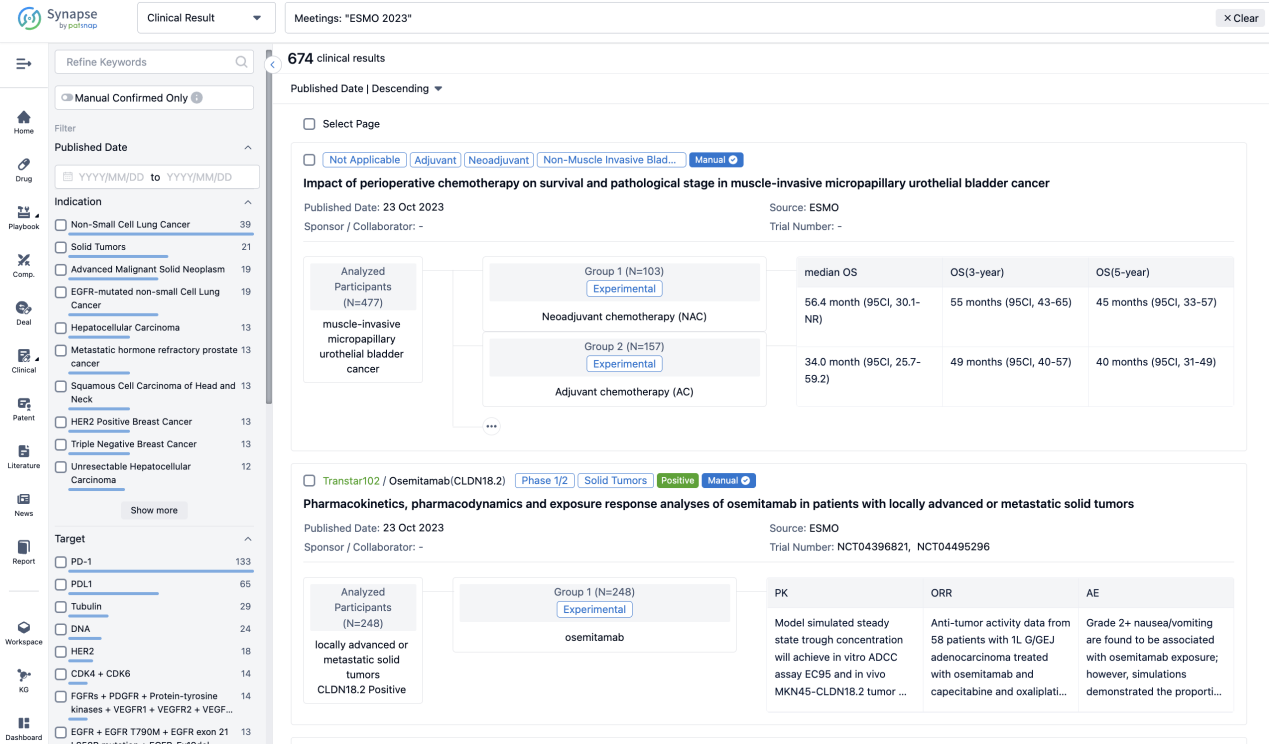
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
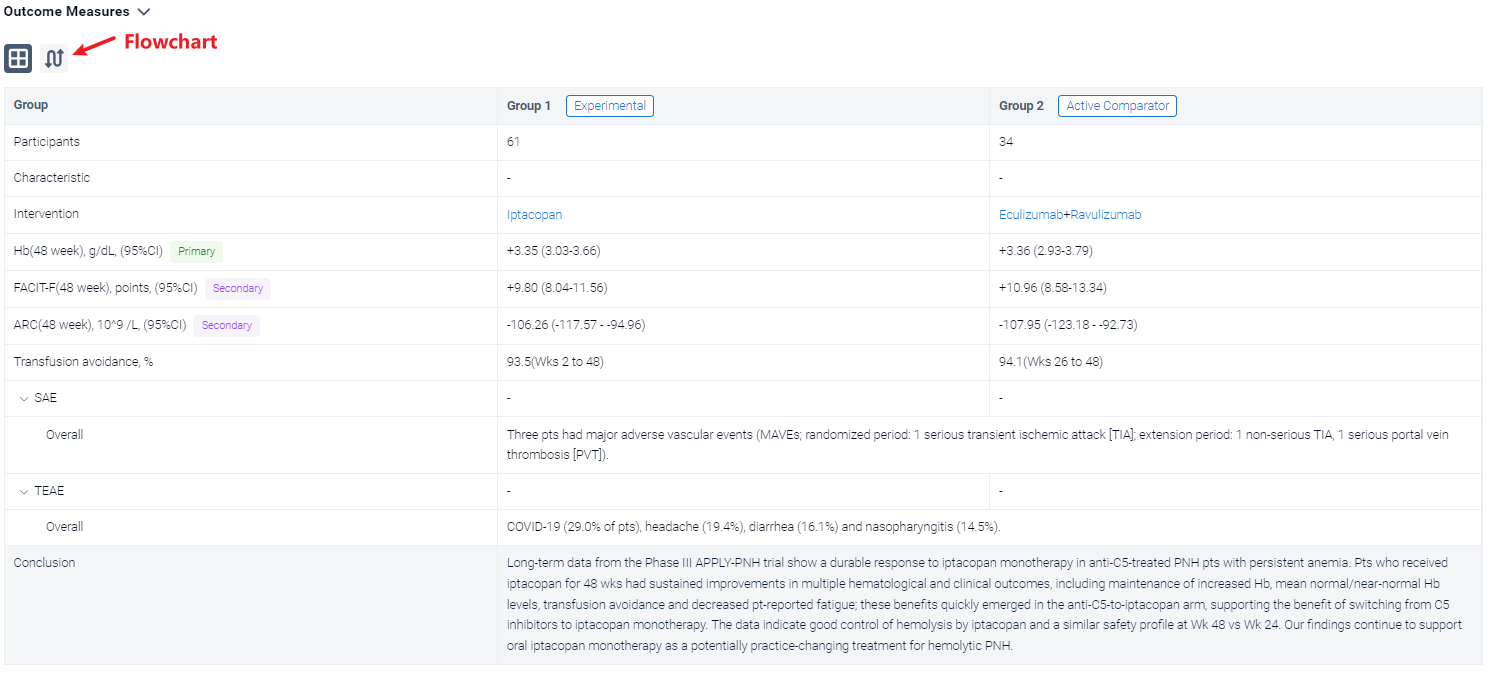
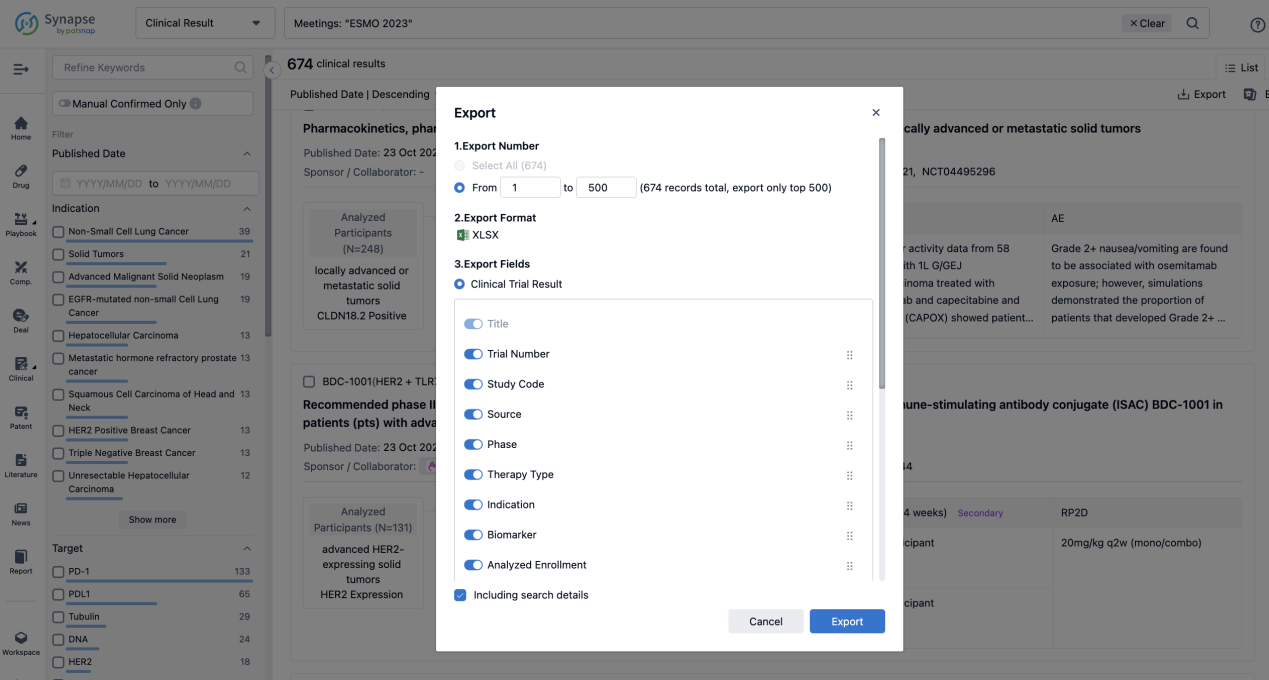
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!