What are KRAS inhibitors and how do you quickly get the latest development progress?
KRAS, or Kirsten rat sarcoma viral oncogene homolog, is a crucial protein involved in cell signaling athways within the human body. It acts as a molecular switch, regulating cell growth, differentiation, and survival. KRAS plays a significant role in transmitting signals from cell surface receptors to the nucleus, influencing various cellular processes. Mutations in the KRAS gene are frequently observed in various cancers, including colorectal, lung, and pancreatic cancers. These mutations lead to the constitutive activation of KRAS, resulting in uncontrolled cell proliferation and tumor growth. Understanding the role of KRAS is essential for developing targeted therapies to inhibit its aberrant activity and combat cancer.
KRAS is one of the earliest discovered cancer genes, and its mutations are common in cancer patients. However, for a long time, the carcinogenic KRAS protein was considered an “undruggable” target. This situation began to change with the approval of the KRAS G12C inhibitors Lumakras (sotorasib) and Krazati (adagrasib). These two drugs target the KRAS G12C mutation, which is one of the many mutations of KRAS, mainly occurring in lung and colorectal cancers. However, scientists have begun to study other types of KRAS mutations, aiming to develop pan-KRAS targeted therapies that can target multiple or even all KRAS mutations. Recently, a paper published in Nature reported a new inhibitor molecule by researchers at the Memorial Sloan Kettering Cancer Center in the United States, which can effectively block more types of KRAS mutations. This new inhibitor molecule, named BI-2865, can selectively bind to the inactive state of the KRAS protein, thereby blocking carcinogenic signals. The advantage of this mechanism of action is that it does not need to form a covalent bond with a specific mutation amino acid, so it is expected to act on multiple KRAS mutation proteins at the same time. Currently, this new inhibitor molecule is undergoing preclinical research and is expected to enter clinical trials within the next year. If successful, it will bring good news to a large number of cancer patients. At the same time, this research also lays a solid foundation for the development of new cancer therapies targeting KRAS and other carcinogenic proteins.
Based on the analysis of the data provided, the current competitive landscape of target KRAS in the pharmaceutical industry is characterized by the involvement of multiple companies at various stages of development. Mirati Therapeutics, Inc., Amgen, Inc., Eli Lilly & Co., Novartis AG, Zai Lab Ltd., Innovent Biologics, Inc., BeiGene Ltd., Roche Holding AG, Jacobio Pharmaceuticals Group Co., Ltd., and other companies are actively engaged in research and development in this area.
Overall, the target KRAS presents a competitive landscape with active research and development efforts from various companies and countries. The future development of target KRAS holds promise for the advancement of treatments for indications such as Non-Small Cell Lung Cancer, Colorectal Cancer, Pancreatic Cancer, and Solid Tumors, among others.
How do they work?
KRAS inhibitors are a type of medication or drug that specifically target and inhibit the activity of KRAS proteins. KRAS is a gene that plays a crucial role in regulating cell growth and division. However, mutations in the KRAS gene can lead to the production of abnormal KRAS proteins that are constantly active, promoting uncontrolled cell growth and contributing to the development of various cancers, including lung, colorectal, and pancreatic cancers.
KRAS inhibitors work by binding to the abnormal KRAS proteins and blocking their signaling pathways, thereby inhibiting their activity and preventing the excessive cell growth associated with cancer. These inhibitors are designed to specifically target the mutant KRAS proteins while sparing the normal, healthy KRAS proteins in the body.
The development of KRAS inhibitors has been a significant advancement in cancer treatment, as KRAS mutations are highly prevalent in certain types of cancer and have historically been challenging to target directly. By inhibiting the activity of mutant KRAS proteins, these inhibitors have the potential to slow down or halt the growth of cancer cells, providing new therapeutic options for patients with KRAS-mutated cancers.
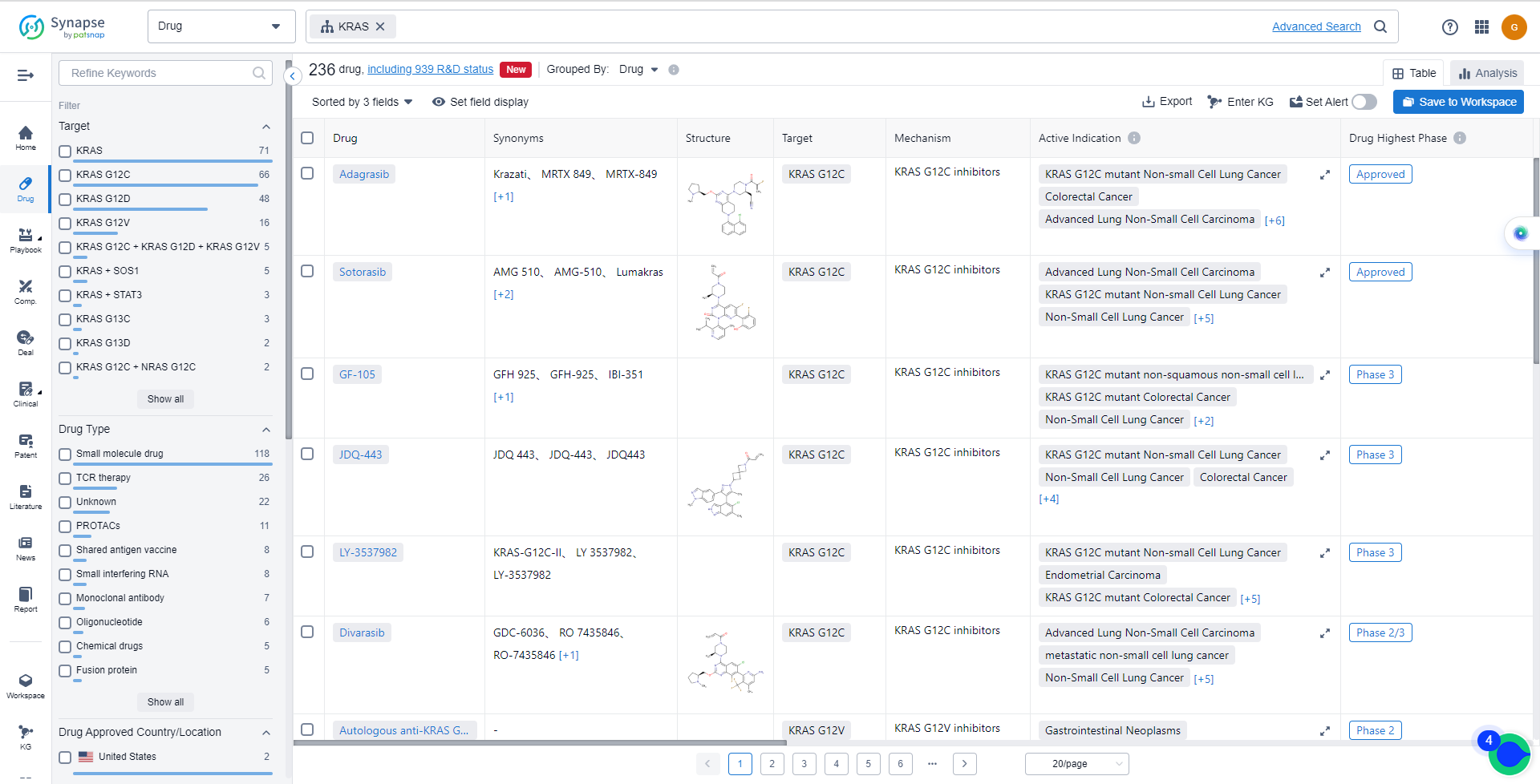
List of KRAS Inhibitors
The currently marketed KRAS inhibitors include:
For more information, please click on the image below.
What are KRAS inhibitors used for?
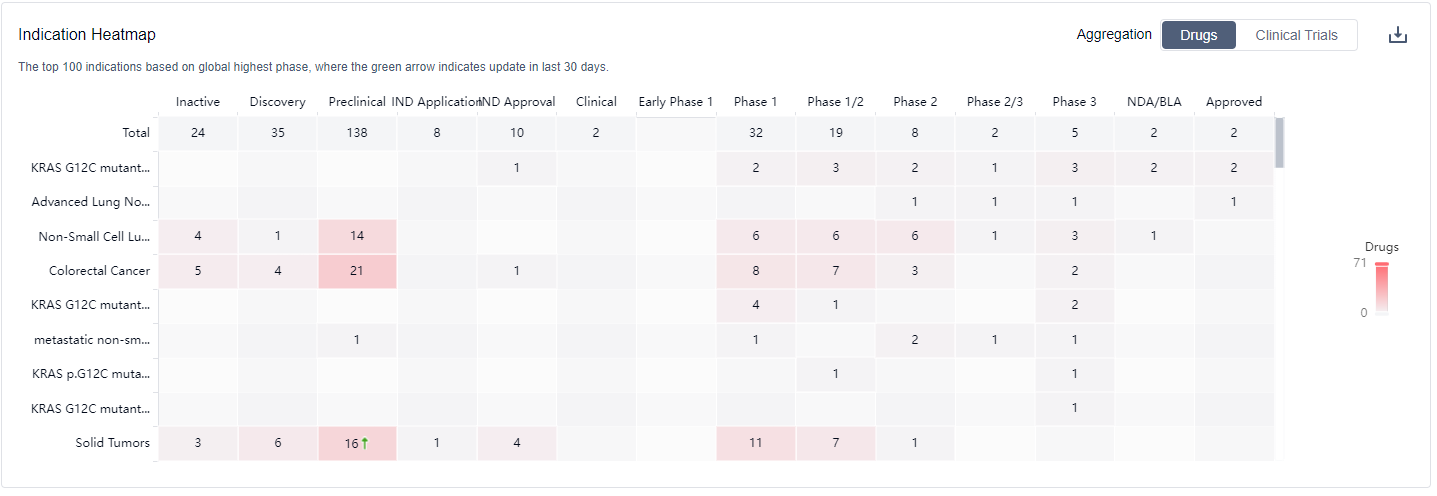
Drugs targeting KRAS have been approved for indications such as KRAS G12C mutant Non-small Cell Lung Cancer, Advanced Lung Non-Small Cell Carcinoma, Non-Small Cell Lung Cancer, Colorectal Cancer, metastatic non-small cell lung cancer, KRAS p.G12C mutant colorectal cancer, KRAS G12C mutant non-squamous non-small cell lung cancer, Solid Tumors, Pancreatic Cancer, Lung Cancer, Renal Cell Carcinoma, Gastrointestinal Neoplasms, Pancreatic Ductal Adenocarcinoma, Neoplasms, KRAS mutation-related tumors, and KRAS G12D mutation Solid Tumors. For more information, please click on the image below to log in and search.
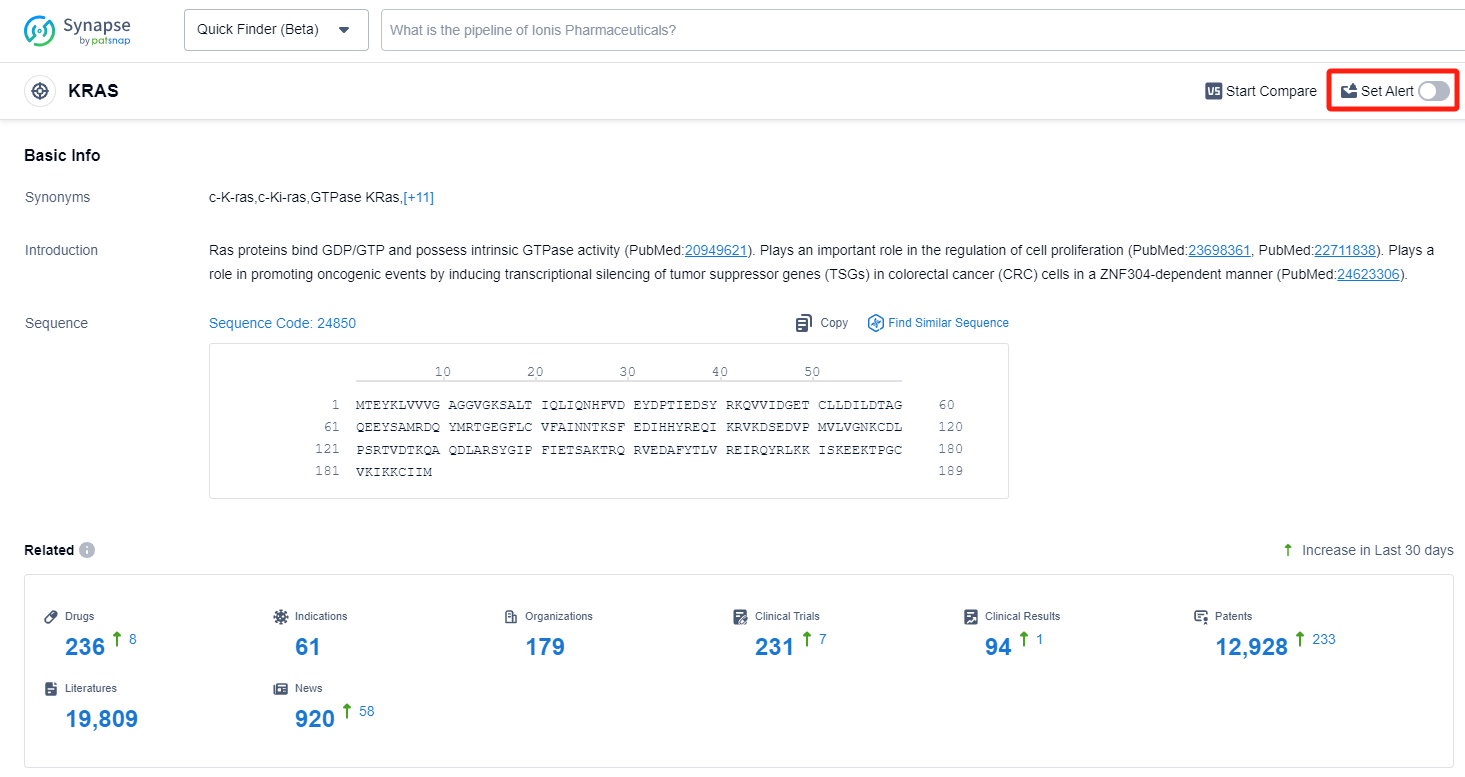
How to obtain the latest development progress of KRAS inhibitors?
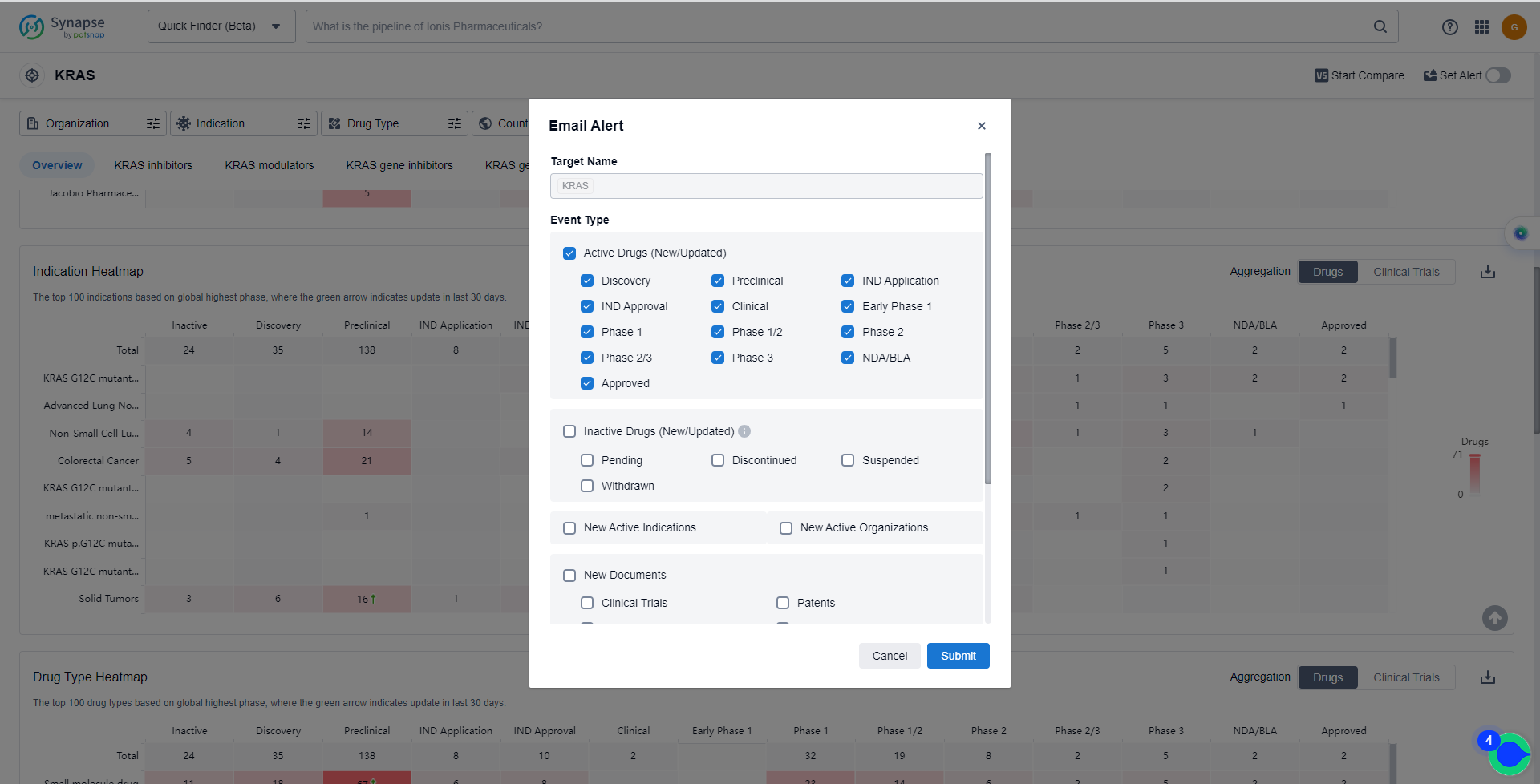
In the Synapse database, you can keep abreast of the latest research and development advances of KRAS inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!