European Commission Approves CStone's Sugemalimab (Cejemly®) for First-Line Non-Small Cell Lung Cancer Treatment
CStone Pharmaceuticals, a biopharmaceutical company emphasizing innovation in anti-cancer treatment research and development, revealed that the European Commission has granted approval for sugemalimab in conjunction with platinum-based chemotherapy as a first-line treatment for adults dealing with metastatic non-small-cell lung cancer. This approval is specifically for patients whose cancer does not exhibit sensitizing EGFR mutations or genomic abnormalities in ALK, ROS1, or RET.
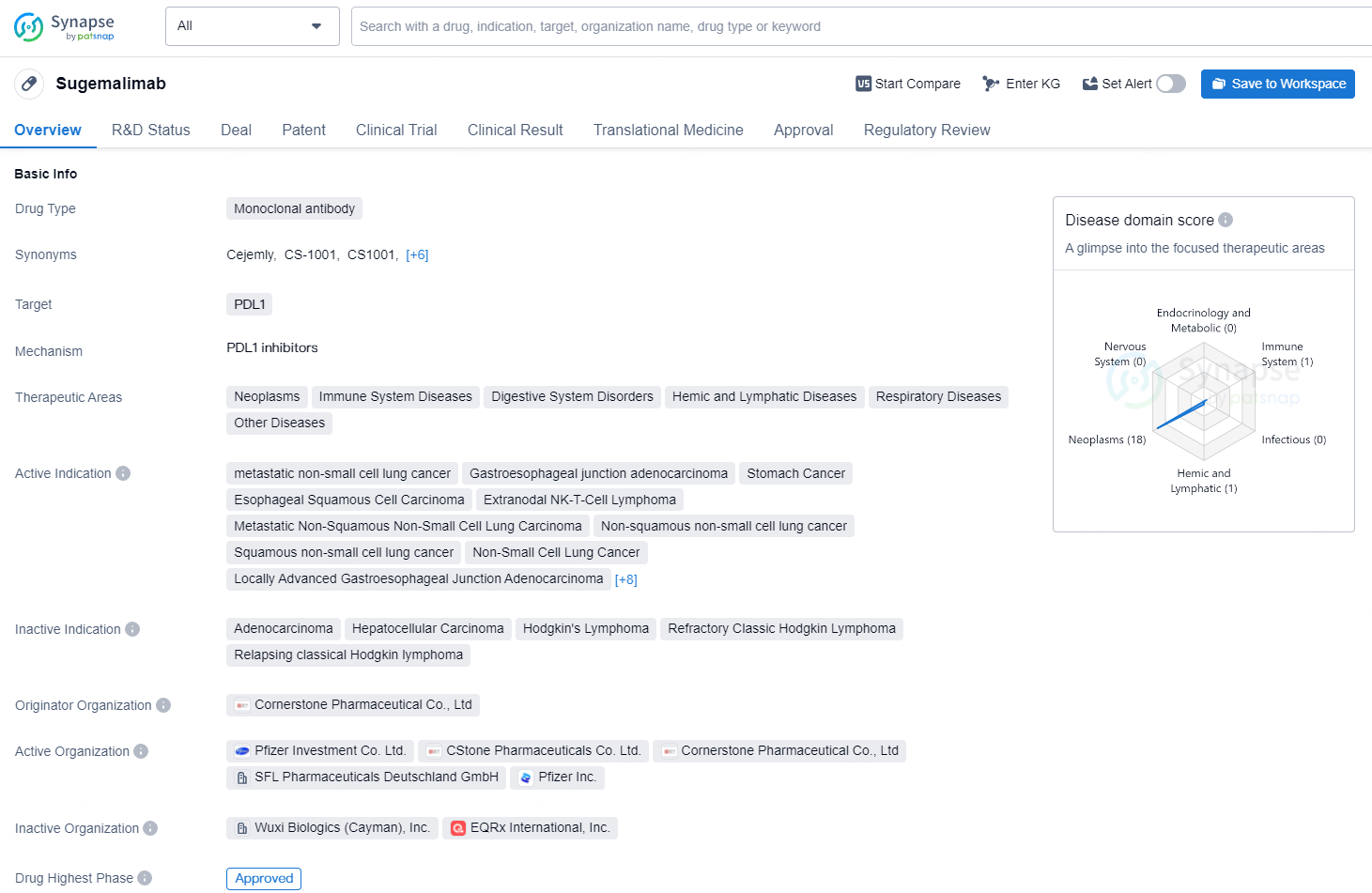
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Sugemalimab has become the inaugural anti-PD-L1 monoclonal antibody sanctioned in Europe for use in conjunction with chemotherapy as a first-line therapy for both squamous and non-squamous NSCLC. This milestone positions CStone as the pioneer innovative biopharmaceutical company to successfully deliver a domestically developed anti-PD-L1 mAb from China to the global marketplace.
The approval from the European Commission is largely based on the findings from the GEMSTONE-302 study—a multicenter, randomized, double-blind phase 3 trial. The study showcased that sugemalimab combined with chemotherapy notably extended progression-free survival and overall survival in comparison to placebo combined with chemotherapy in treatment-naïve patients with metastatic NSCLC.
These results have been documented in The Lancet Oncology and Nature Cancer and presented at several international academic conferences. Moreover, long-term treatment and survival data from the GEMSTONE-302 study will be featured in a poster session at the 2024 ESMO Annual Meeting.
Dr. Jason Yang, CEO, President of R&D, and Executive Director of the Board at CStone, remarked, “Today's announcement is a landmark achievement in CStone's aspiration to become a global leader dedicated to cancer eradication. Sugemalimab has not only earned its place as CStone’s first self-developed product to gain overseas marketing approval but also stands as the world’s first anti-PD-L1 mAb to be approved in Europe in combination with chemotherapy for both types of NSCLC as a first-line treatment.”
Dr. Jason Yang highlighted, “The international sanction and commercial launch of sugemalimab underscore a critical milestone in our Pipeline 1.0 strategy, underscoring our prowess in developing forefront immuno-oncology drugs both for monotherapy and as a cornerstone for combination therapies. In the Pipeline 2.0 strategy, we hold global rights to a plethora of highly promising candidates, either in international multicenter clinical trials or nearing the clinical stage, with the potential to be first-in-class or best-in-class.”
Dr. Yang further added, “The seven-year journey to establish sugemalimab as a first-line treatment for NSCLC in Europe and other cancer types in China epitomizes the rich expertise of numerous Chinese oncology experts. We feel privileged and humbled that this ‘Chinese innovative solution’ might significantly enhance outcomes for lung cancer patients worldwide, providing both extended survival and an improved quality of life.”
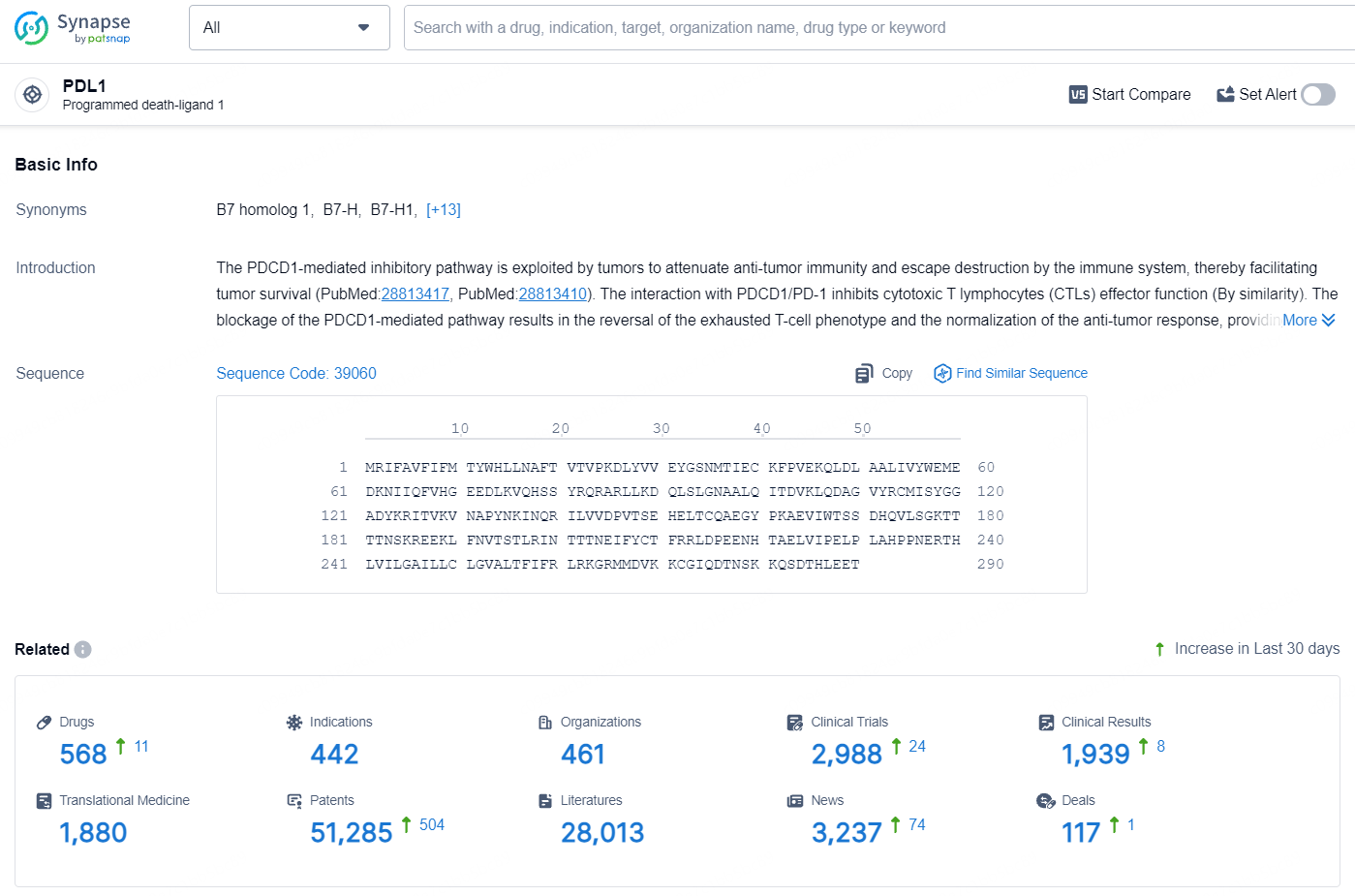
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 1, 2024, there are 568 investigational drugs for the PDL1 targets, including 442 indications, 461 R&D institutions involved, with related clinical trials reaching 2988, and as many as 51285 patents.
Sugemalimab is an important addition to the field of biomedicine, particularly for the treatment of various cancers and other related diseases. The drug's approvals and designations demonstrate its potential as a significant therapeutic option for patients in need. Furthermore, the monoclonal antibody's targeting of PDL1 represents a promising approach in the development of innovative treatments for a broad range of medical conditions. The drug's diverse indications and regulatory status indicate its potential to address unmet medical needs and make a substantial impact on patient care in the pharmaceutical industry.