Nectin Therapeutics Licenses Novel Antibodies to Immunome
Nectin Therapeutics, Ltd., a biotechnology firm specializing in innovative immunotherapies and antibody drug conjugates aimed at overcoming tumor resistance, disclosed a worldwide, exclusive licensing deal with Immunome Inc., a biotech company dedicated to creating top-tier targeted cancer treatments. According to the agreement, Immunome has obtained exclusive rights to a collection of antibodies directed at an undisclosed target.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"This pact enables Nectin to extract value while continuing to hone in on our pioneering anti-PVR program (NTX1088) via its ongoing clinical trials in cancers with substantial unmet needs, and to progress our innovative ADCs into clinical trials. Immunome’s leadership team’s proven expertise in developing and commercializing groundbreaking ADC therapies makes it an exceptional collaborator to drive these valuable assets forward," stated Fabian Tenenbaum, CEO of Nectin Therapeutics.
"Immunome believes that the future generation of groundbreaking antibody-drug conjugates will target novel sites by combining superior quality antibodies with advanced linker-payload technology," said Clay Siegall, PhD, President and CEO of Immunome. "We value the progress Nectin has achieved with these antibodies and anticipate continuing their development."
According to the terms of the pact, Nectin has awarded Immunome an exclusive, global, all-fields license for monoclonal antibodies targeting a single undisclosed antigen. Immunome will handle the research, development, production, and marketing of products including these antibodies. Nectin will receive an initial payment and will be eligible for milestone-based payments and royalties..
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
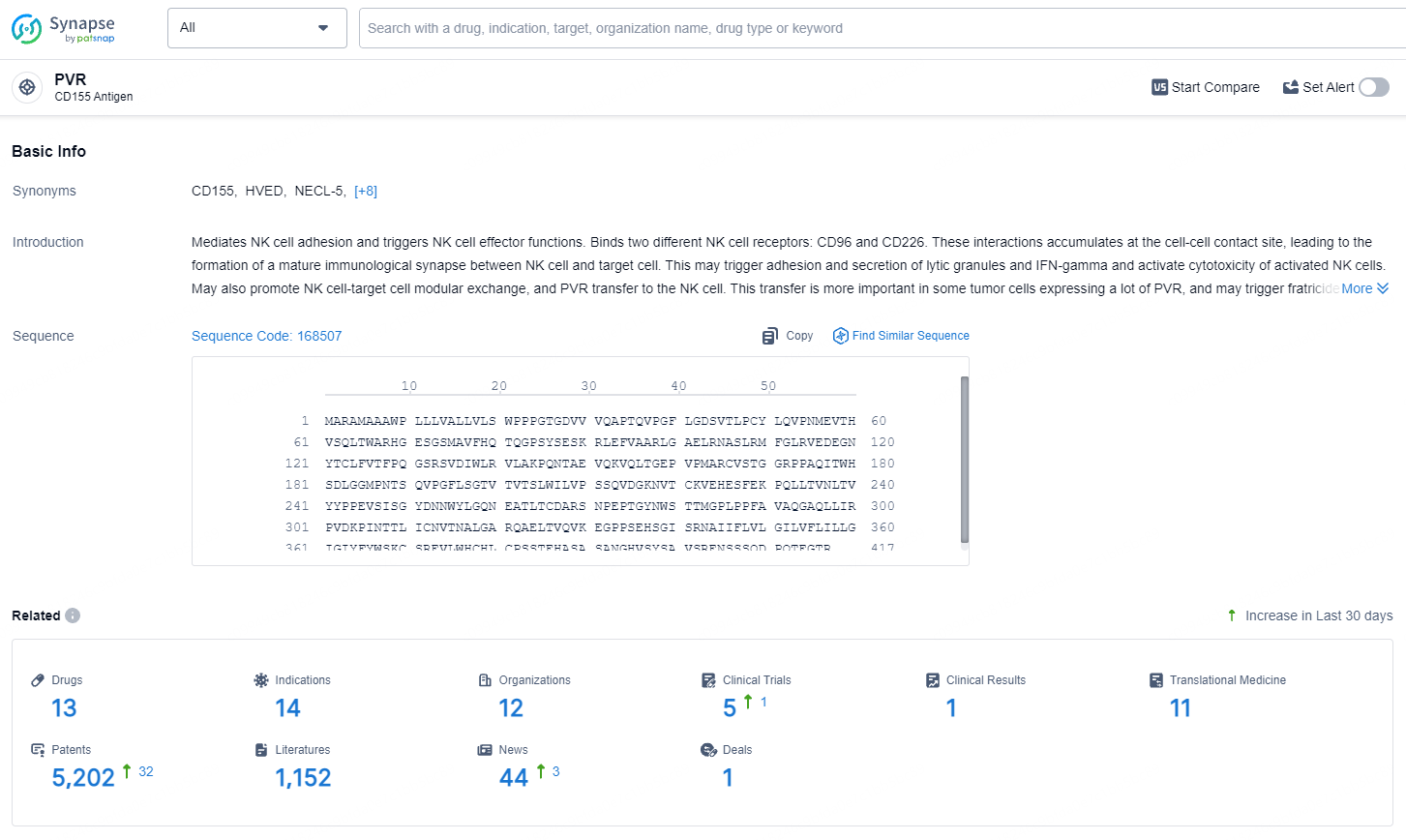
According to the data provided by the Synapse Database, As of August 1, 2024, there are 13 investigational drugs for the PVR target, including 14 indications, 12 R&D institutions involved, with related clinical trials reaching 5, and as many as 5202 patents.
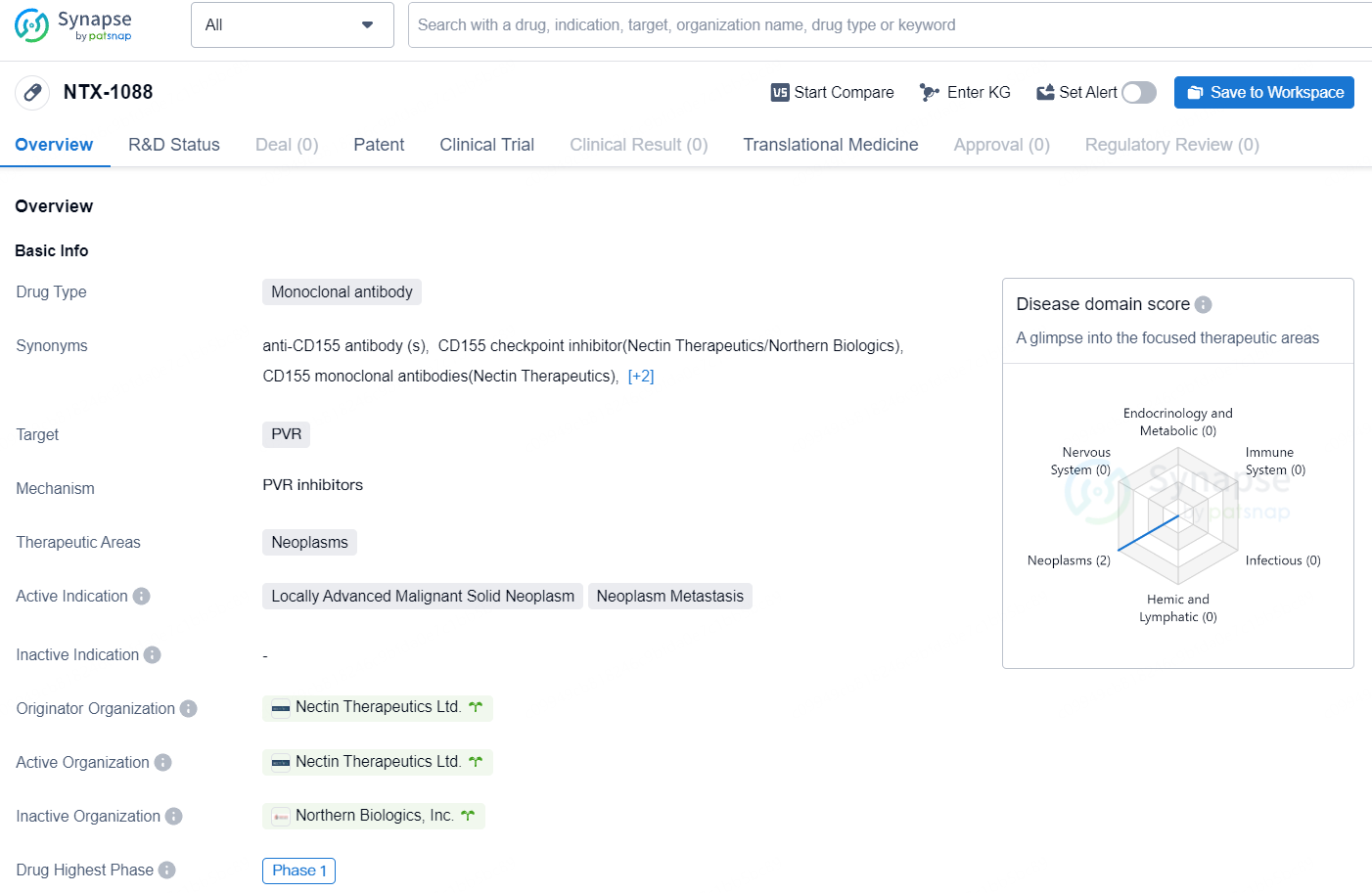
NTX-1088 is a monoclonal antibody drug developed by Nectin Therapeutics Ltd. with a focus on targeting PVR in the treatment of neoplasms, specifically locally advanced malignant solid neoplasms and neoplasm metastasis. As of now, the drug has reached Phase 1 in its global development, indicating its progression through initial human testing.