Is Vilobelimab approved by the FDA?
Vilobelimab, marketed under the brand name Gohibic, has been authorized by the FDA for emergency use but has not yet received full FDA approval for the treatment of COVID-19.
Vilobelimab is used to treat severe COVID-19 in hospitalized adults. It is specifically authorized for use when treatment is initiated within 48 hours of receiving invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO).
Emergency Use Authorization (EUA)
The FDA has granted an Emergency Use Authorization (EUA) for vilobelimab for the treatment of COVID-19 under specific conditions:
- Conditions of Use: The treatment must be started within 48 hours of the patient being intubated or placed on ECMO.
- Dosage: 800 mg IV for up to 6 doses. The dosing schedule is Day 1 (within 48 hours of intubation), followed by Days 2, 4, 8, 15, and 22 as long as the patient remains hospitalized.
Side Effects
Common Side Effects
- Confusion
- Constipation
- Rash
- Irregular heartbeats
- Pain, swelling, or warmth in one leg
- Pain and burning when urinating
- Chest pain, breathing problems, shortness of breath
- Severe headache, blurred vision, pounding in your neck or ears
- Easy bruising, unusual bleeding, purple or red spots under the skin
- Cold symptoms (stuffy nose, sneezing, sore throat)
- Symptoms of sepsis (confusion, fever, chills, severe drowsiness, fast heartbeats, rapid breathing, feeling very ill)
- Symptoms of herpes virus (cold sores around the mouth, skin sores or blisters, itching, tingling, burning pain in thigh or lower back)
Serious Side Effects
Seek immediate medical help if experiencing signs of an allergic reaction, such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat. Serious side effects may include signs of infection like fever, chills, sore throat, body aches, unusual tiredness, loss of appetite, bruising, or bleeding.
Warnings and Precautions
- Allergies: Inform your doctor about any known allergies.
- Other Infections: Notify your doctor if you have any other infections besides COVID-19.
- Pregnancy and Breastfeeding: Inform your doctor if you are pregnant, planning to become pregnant, or breastfeeding.
Administration
Vilobelimab is administered by a healthcare provider in a hospital setting. Follow all instructions and medical guidance during treatment.
Drug Interactions
Other medications, including prescription and over-the-counter drugs, vitamins, and herbal products, may interact with vilobelimab. It is important to inform your doctor about all medications you are taking.
Conclusion
While vilobelimab (Gohibic) has not received full FDA approval, it has been granted an EUA for the treatment of severe COVID-19 in specific circumstances. This authorization allows its use in hospitalized patients within 48 hours of IMV or ECMO initiation, providing a critical treatment option during the pandemic.
How to obtain the latest development progress of all drugs?
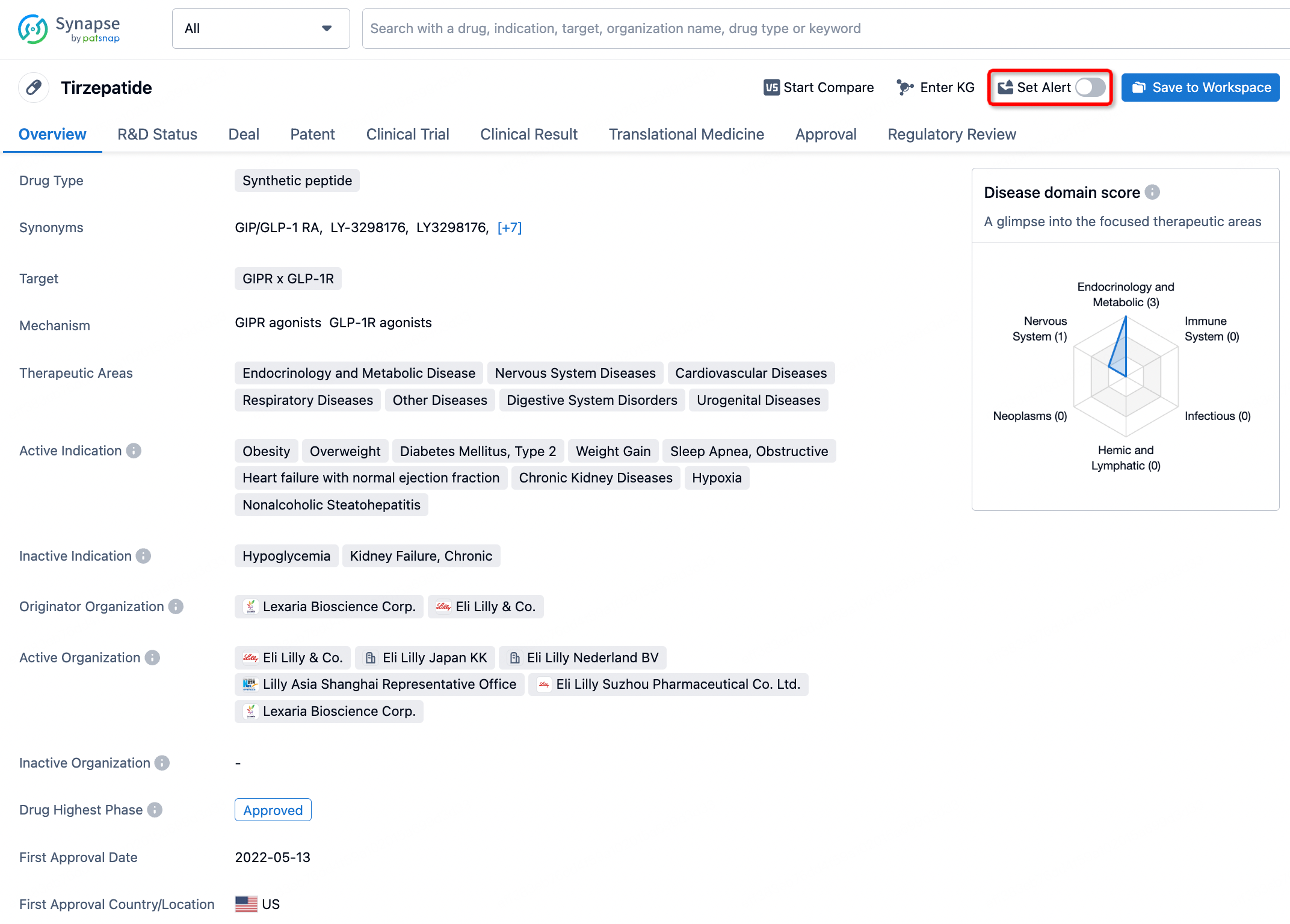
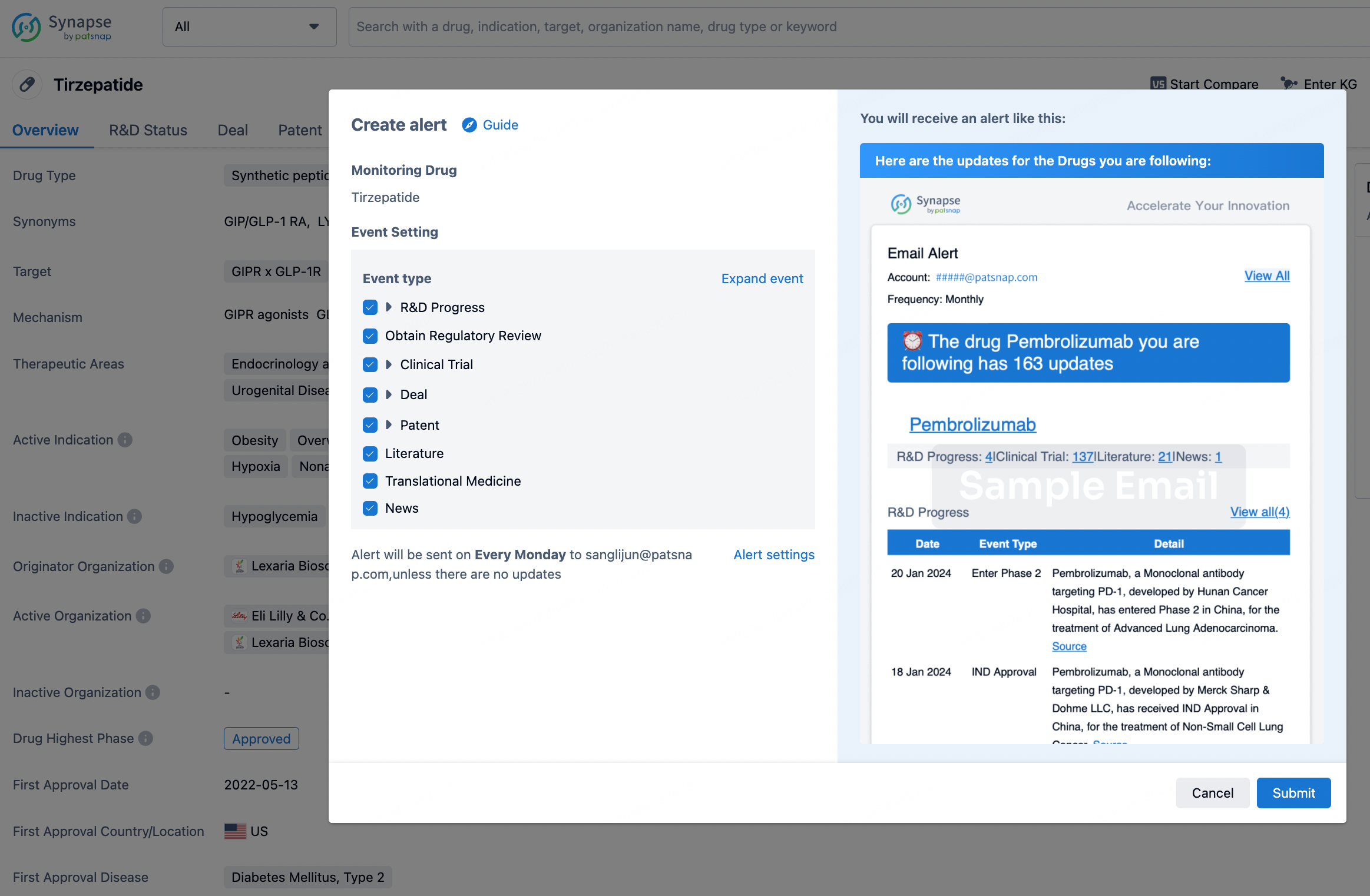
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!