European Medicines Agency Reviews Bristol Myers Squibb's CAR T Therapy Breyanzi for Follicular Lymphoma
Bristol Myers Squibb (NYSE: BMY) disclosed that the European Medicines Agency (EMA) has accepted its Type II variation application aimed at broadening the indication for Breyanzi® (lisocabtagene maraleucel; liso-cel), a CD19-targeted chimeric antigen receptor (CAR) T cell therapy. The new indication includes treatment for adult patients with relapsed or refractory follicular lymphoma (FL) who have undergone at least two prior systemic therapies. Acceptance of the application confirms the submission's completeness and initiates the scientific evaluation under the EMA’s centralized review process.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 "Follicular lymphoma affects numerous individuals, especially those with relapsed or refractory cases who often experience diminishing treatment efficacy with each subsequent therapy," remarked Anne Kerber, senior vice president and head of Late Clinical Development at Bristol Myers Squibb's Hematology, Oncology, and Cell Therapy (HOCT) division. "Breyanzi is a unique CAR T cell therapy, and we are eager to collaborate with the European Medicines Agency to make this vital treatment available to patients with relapsed or refractory follicular lymphoma, with the aim of enhancing outcomes and achieving lasting remission."
"Follicular lymphoma affects numerous individuals, especially those with relapsed or refractory cases who often experience diminishing treatment efficacy with each subsequent therapy," remarked Anne Kerber, senior vice president and head of Late Clinical Development at Bristol Myers Squibb's Hematology, Oncology, and Cell Therapy (HOCT) division. "Breyanzi is a unique CAR T cell therapy, and we are eager to collaborate with the European Medicines Agency to make this vital treatment available to patients with relapsed or refractory follicular lymphoma, with the aim of enhancing outcomes and achieving lasting remission."
The application is backed by findings from the Phase 2 TRANSCEND FL study, the largest clinical trial to date investigating a CAR T cell therapy in patients with relapsed or refractory indolent non-Hodgkin lymphoma (NHL), including follicular lymphoma (FL). The study included adults with relapsed or refractory FL who received Breyanzi in the high-risk second-line and third-line plus settings. In this trial, Breyanzi showed a high overall response rate, the primary endpoint, with responses being both profound and durable. The safety profile of Breyanzi in follicular lymphoma aligns with the established and manageable safety observations across clinical studies.
Follicular lymphoma is the second most prevalent type of NHL, representing 20 to 30 percent of all NHL cases. Historically, FL has been seen as an incurable condition, with patients often experiencing relapse after initial therapy and progressively worsening prognosis with each recurrence. Despite therapeutic advancements, there is still a significant need for additional treatment options that provide extended treatment-free intervals with durable, complete responses.
Currently, Breyanzi is approved in the European Union for treating adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma (HGBCL), primary mediastinal large B-cell lymphoma (PMBCL), and FL grade 3B (FL3B), for those who relapsed within 12 months from finishing, or are refractory to, first-line chemoimmunotherapy, and for the treatment of relapsed or refractory DLBCL, PMBCL, and FL3B after two or more lines of systemic therapy.
The Japanese Ministry of Health, Labour and Welfare has also approved a supplemental New Drug Application for Breyanzi for treating relapsed or refractory follicular lymphoma after one prior line of systemic therapy in patients with high-risk FL and after two or more lines of systemic therapy. This approval, based on the results of the TRANSCEND FL study, makes it the first CAR T therapy approved in the second-line setting globally and marks the third approval for Breyanzi in Japan.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
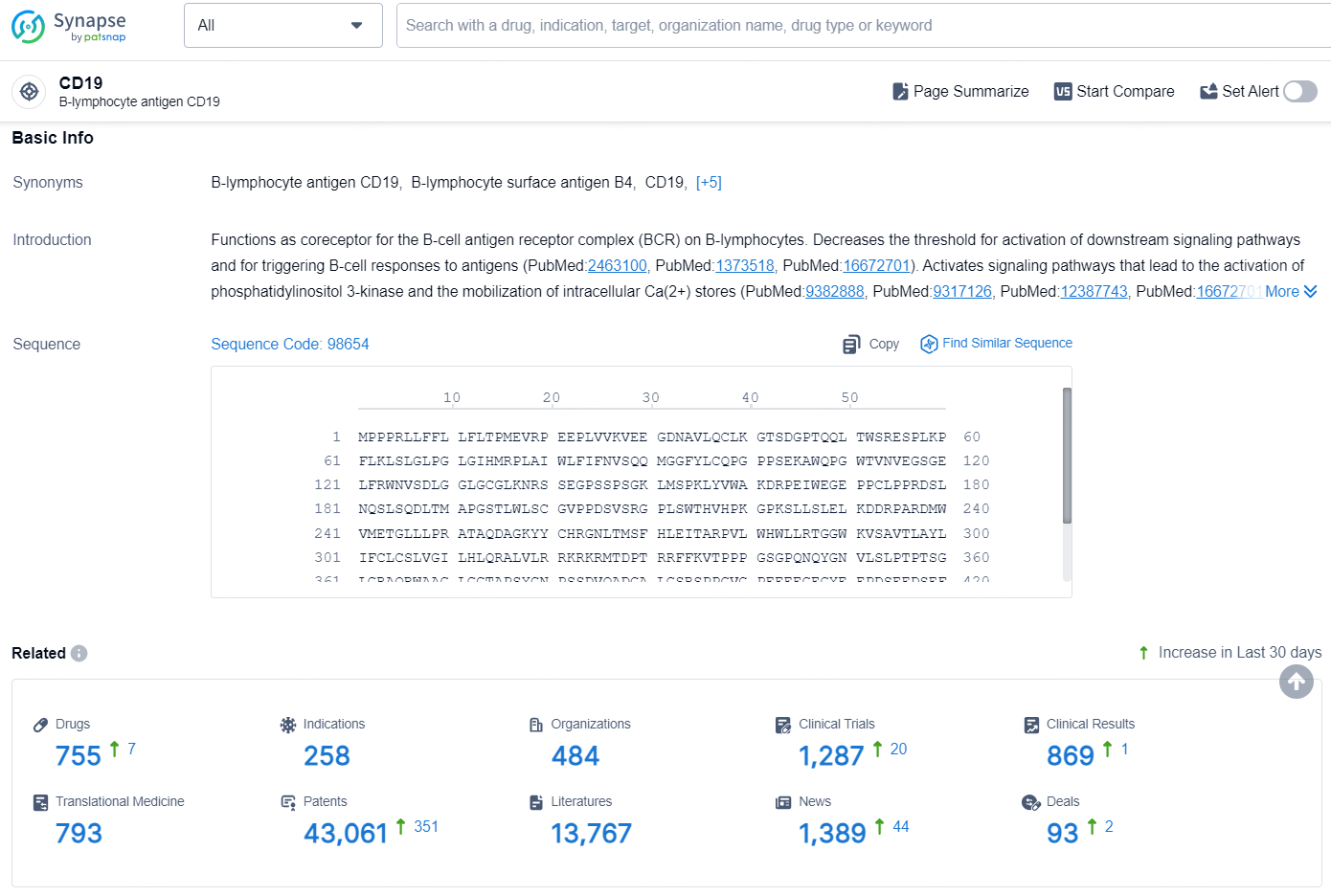
According to the data provided by the Synapse Database, As of August 21, 2024, there are 755 investigational drugs for the CD19 targets, including 258 indications, 484 R&D institutions involved, with related clinical trials reaching 1287, and as many as 43061 patents.
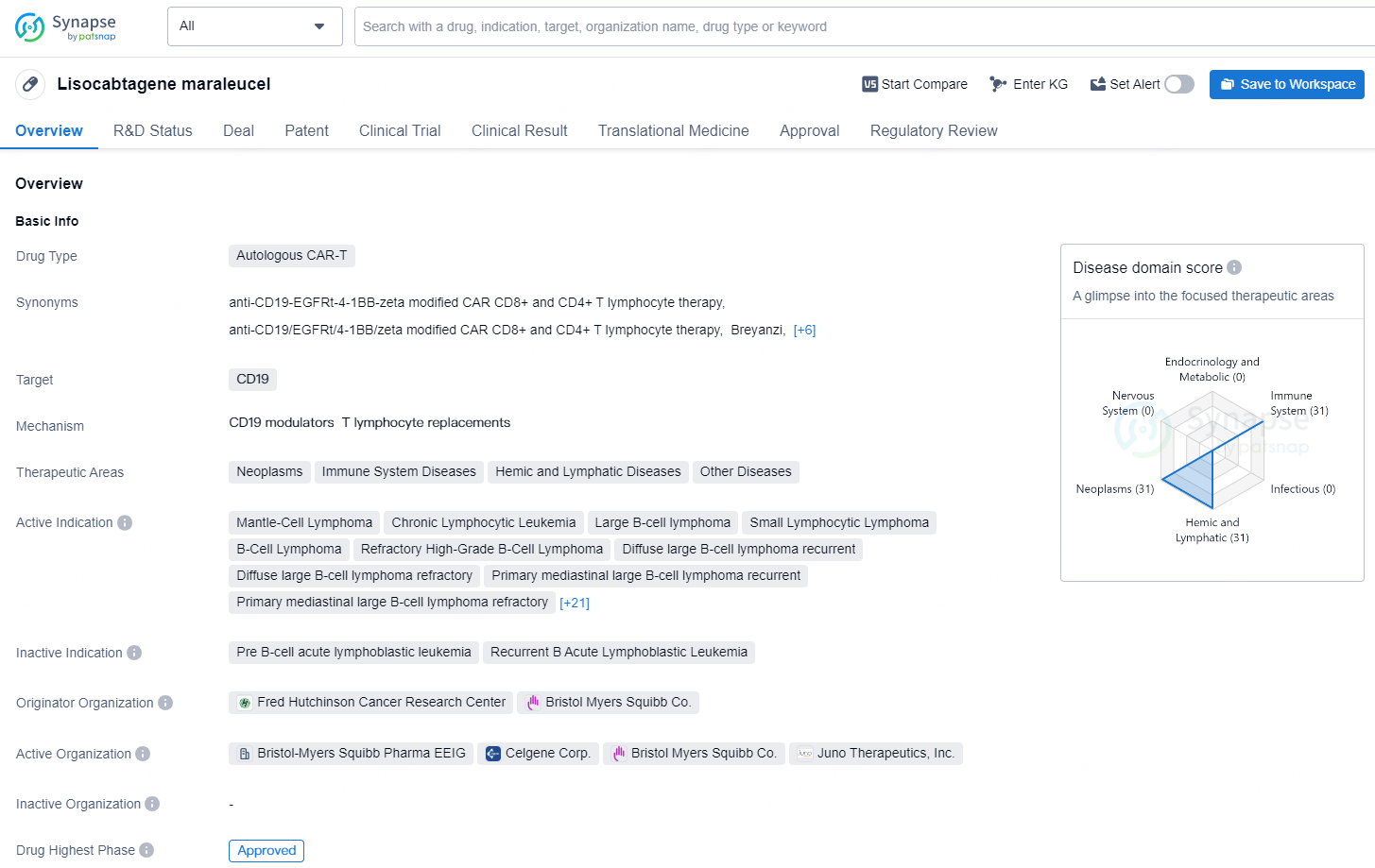
Lisocabtagene maraleucel is a type of Autologous Chimeric Antigen Receptor T-Cell (CAR-T) therapy that targets CD19. It falls within the therapeutic areas of neoplasms, immune system diseases, hemic and lymphatic diseases, and other diseases. The drug has been approved for various indications including Mantle-Cell Lymphoma, Chronic Lymphocytic Leukemia, Large B-cell lymphoma, Small Lymphocytic Lymphoma, B-Cell Lymphoma, Refractory High-Grade B-Cell Lymphoma, Diffuse large B-cell lymphoma recurrent, Diffuse large B-cell lymphoma refractory, Primary mediastinal large B-cell lymphoma recurrent, Primary mediastinal large B-cell lymphoma refractory, Recurrent Grade 3b Follicular Lymphoma, Refractory Grade 3b Follicular Lymphoma, among others.