MOMA Therapeutics Begins Phase 1 Trial for New Polymerase Theta Helicase Inhibitor, MOMA-313
MOMA Therapeutics, a biopharmaceutical company in its clinical stage focusing on the discovery and development of advanced precision medicines, has reported the administration of the initial dose to the first participant in its Phase 1 clinical trial. This trial aims to evaluate the safety and tolerability of MOMA-313, an innovative and highly potent, selective oral inhibitor targeting polymerase theta helicase.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
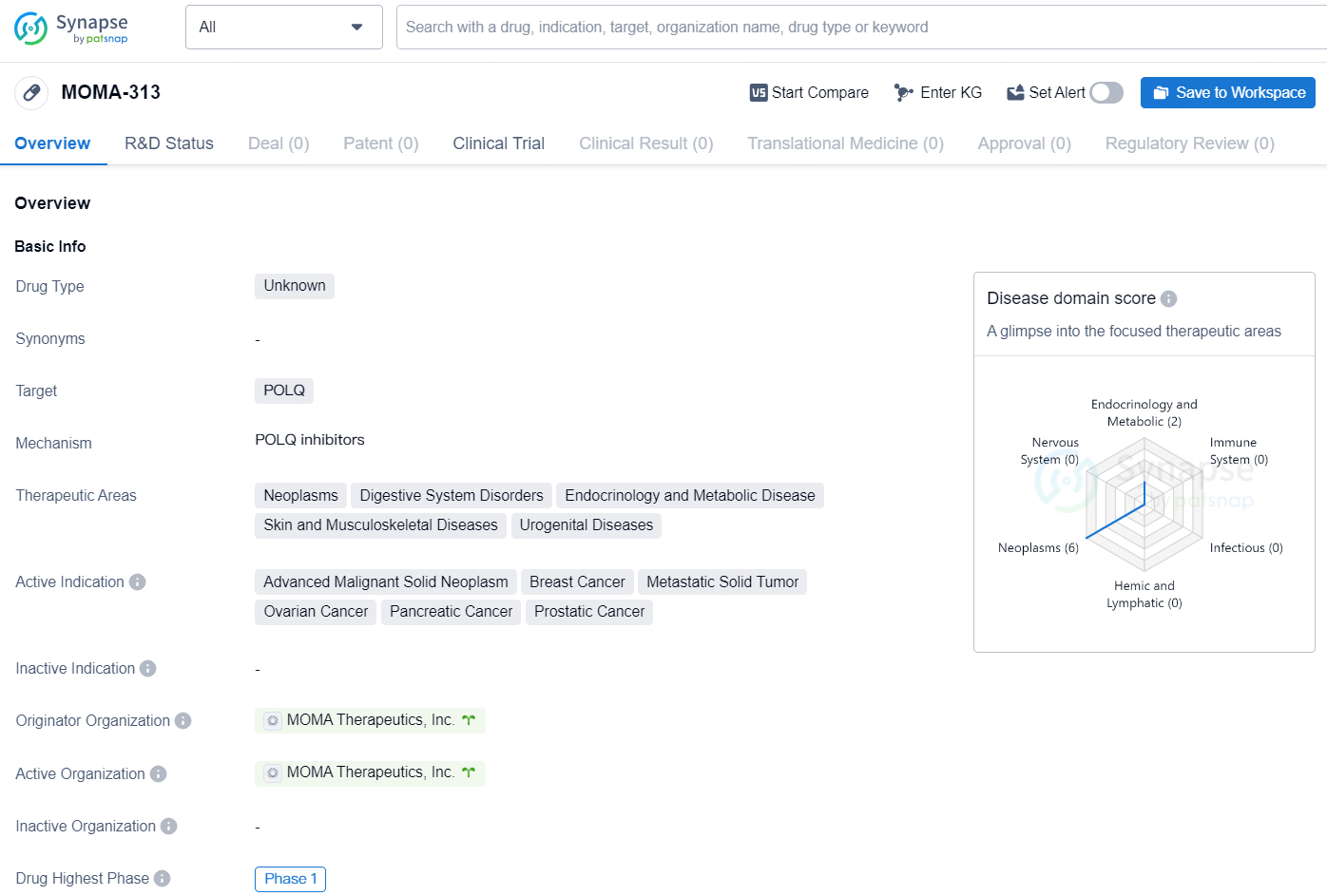 MOMA-313 is being explored for use alongside a PARP inhibitor specifically targeting patients with solid tumors that exhibit alterations in various DNA repair genes, including certain subsets of prostate cancer, pancreatic cancer, and breast cancer.
MOMA-313 is being explored for use alongside a PARP inhibitor specifically targeting patients with solid tumors that exhibit alterations in various DNA repair genes, including certain subsets of prostate cancer, pancreatic cancer, and breast cancer.
Simultaneously, MOMA has announced the selection of a candidate for development under its second main program, MOMA-341, an oral, potent, and selective covalent Werner helicase inhibitor aimed at treating cancers characterized by microsatellite instability. The filing of an IND application with the FDA is expected in the first quarter of 2025.
"We are thrilled to advance two molecules with best-in-class potential, significantly impacting the lives of cancer patients in need," stated Asit Parikh, M.D., Ph.D., CEO of MOMA. "Progressing into clinical development from mere conceptualization a few years ago is truly humbling. This achievement underscores the excellence and unwavering dedication of the entire MOMA team."
"Taking two highly potent and selective drug candidates from our proprietary KNOMATIC platform toward clinical trials is a unique opportunity," added Peter Hammerman, M.D., Ph.D., Chief Scientific Officer of MOMA. "Our aim with each candidate is to turn pioneering science into transformative, life-changing treatments."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
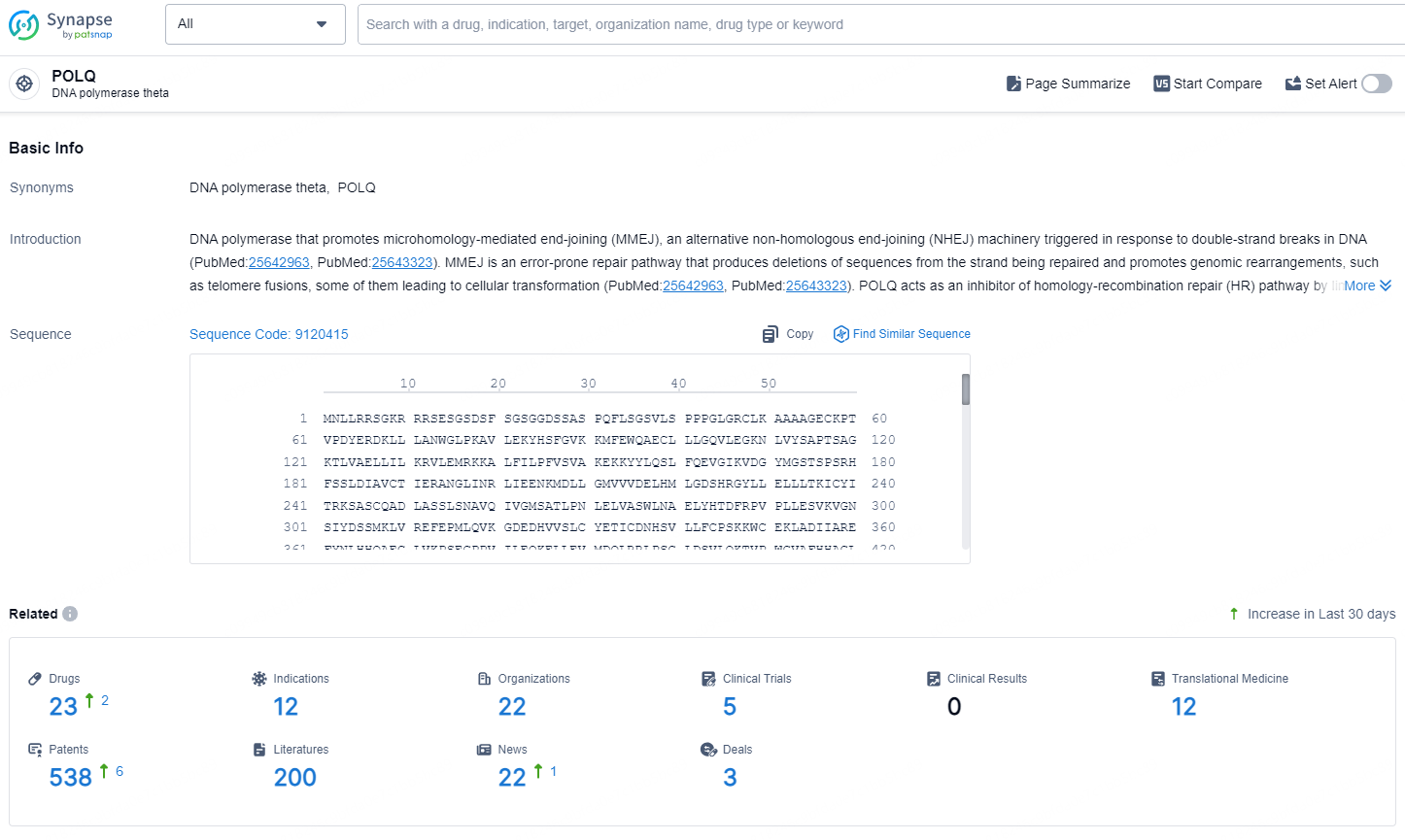
According to the data provided by the Synapse Database, As of August 21, 2024, there are 23 investigational drugs for the POLQ targets, including 12 indications, 22 R&D institutions involved, with related clinical trials reaching 5, and as many as 538 patents.
MOMA-313 is a drug of unknown type that targets the POLQ protein. It is being developed for the treatment of various therapeutic areas including neoplasms, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases. The drug is actively indicated for advanced malignant solid neoplasm, breast cancer, metastatic solid tumor, ovarian cancer, pancreatic cancer, and prostatic cancer.