Satellos Reveals Approval for Initiating Phase 1 Trial of SAT-3247
Satellos Bioscience Inc. (“Satellos” or the “Company”) (TSX: MSCL, OTCQB: MSCLF), a publicly traded biotechnology firm focusing on innovative small molecule treatments for muscle-related diseases and conditions, announced the submission and acceptance of a clinical research proposal to a Human Research Ethics Committee (HREC) in Australia. The proposal seeks regulatory approval under the Therapeutic Goods Administration’s (TGA’s) Clinical Trial Notification (CTN) scheme to initiate a first-in-human Phase 1 clinical trial for SAT-3247.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 "Obtaining approval to start the clinical development of SAT-3247 marks a pivotal milestone for Satellos as we progress with the first small molecule drug capable of restoring the disrupted innate muscle regeneration and repair mechanism in individuals with Duchenne," stated Frank Gleeson, CEO and Co-founder of Satellos. "We are thrilled to move SAT-3247 into initial human trials and embark on this crucial phase in developing an oral medication which we believe could modify the disease."
"Obtaining approval to start the clinical development of SAT-3247 marks a pivotal milestone for Satellos as we progress with the first small molecule drug capable of restoring the disrupted innate muscle regeneration and repair mechanism in individuals with Duchenne," stated Frank Gleeson, CEO and Co-founder of Satellos. "We are thrilled to move SAT-3247 into initial human trials and embark on this crucial phase in developing an oral medication which we believe could modify the disease."
The Phase 1 clinical trial will consist of two segments. The first segment will enroll 72 healthy participants in a blinded, randomized, placebo-controlled, staggered, parallel design to evaluate the safety and pharmacokinetic features of SAT-3247. Participants will be divided among 5 single-ascending dose (SAD) cohorts, 4 multiple-ascending dose (MAD) cohorts, and one food effect (FE) dose cohort. In the second segment, 10 adult volunteers with genetically confirmed DMD will be included in a 28-day, open-label dose cohort to compare safety and pharmacokinetic characteristics with the healthy participant data and investigate pharmacodynamic markers.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
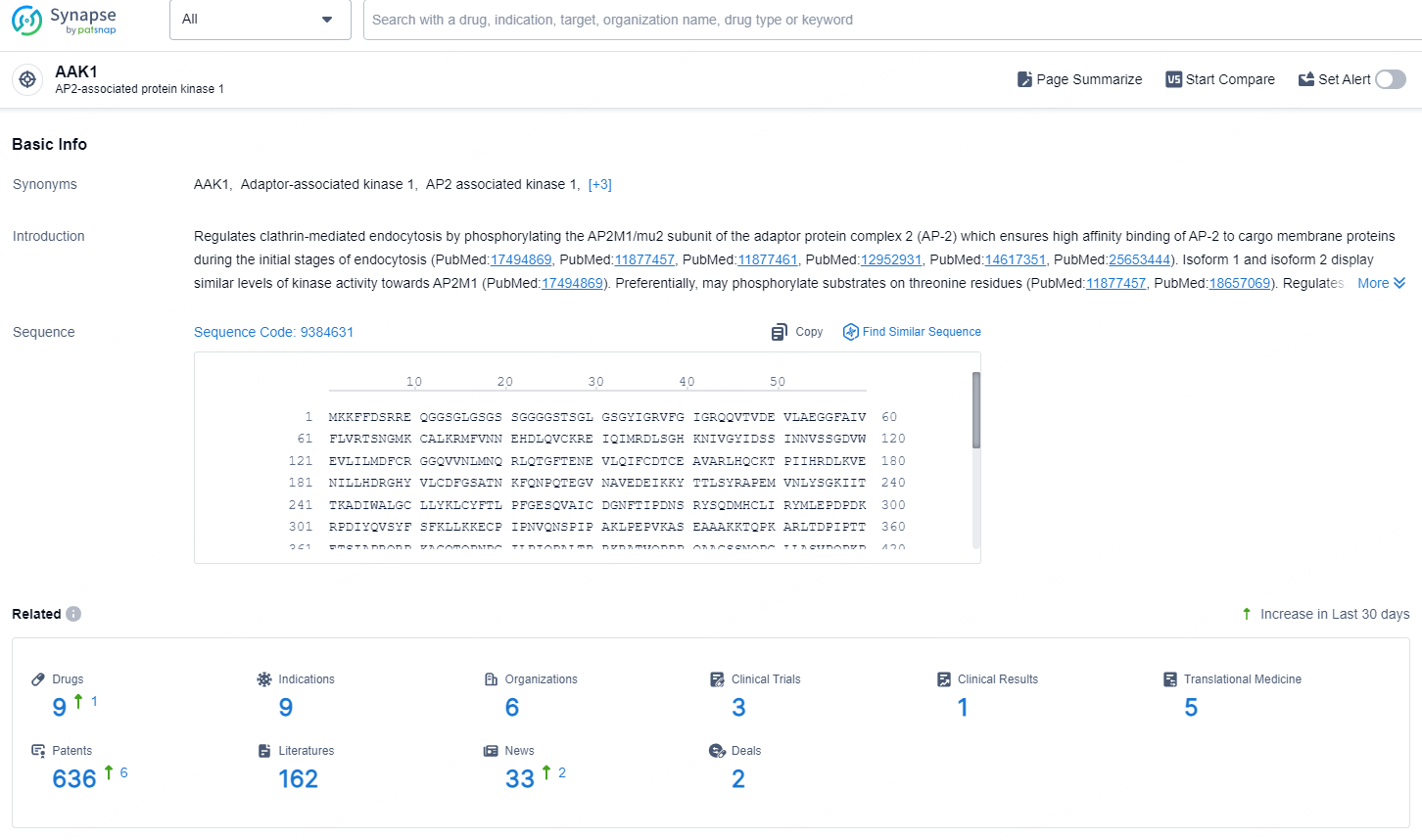
According to the data provided by the Synapse Database, As of August 21, 2024, there are 9 investigational drugs for the AAK1 targets, including 9 indications, 6 R&D institutions involved, with related clinical trials reaching 3, and as many as 636 patents.
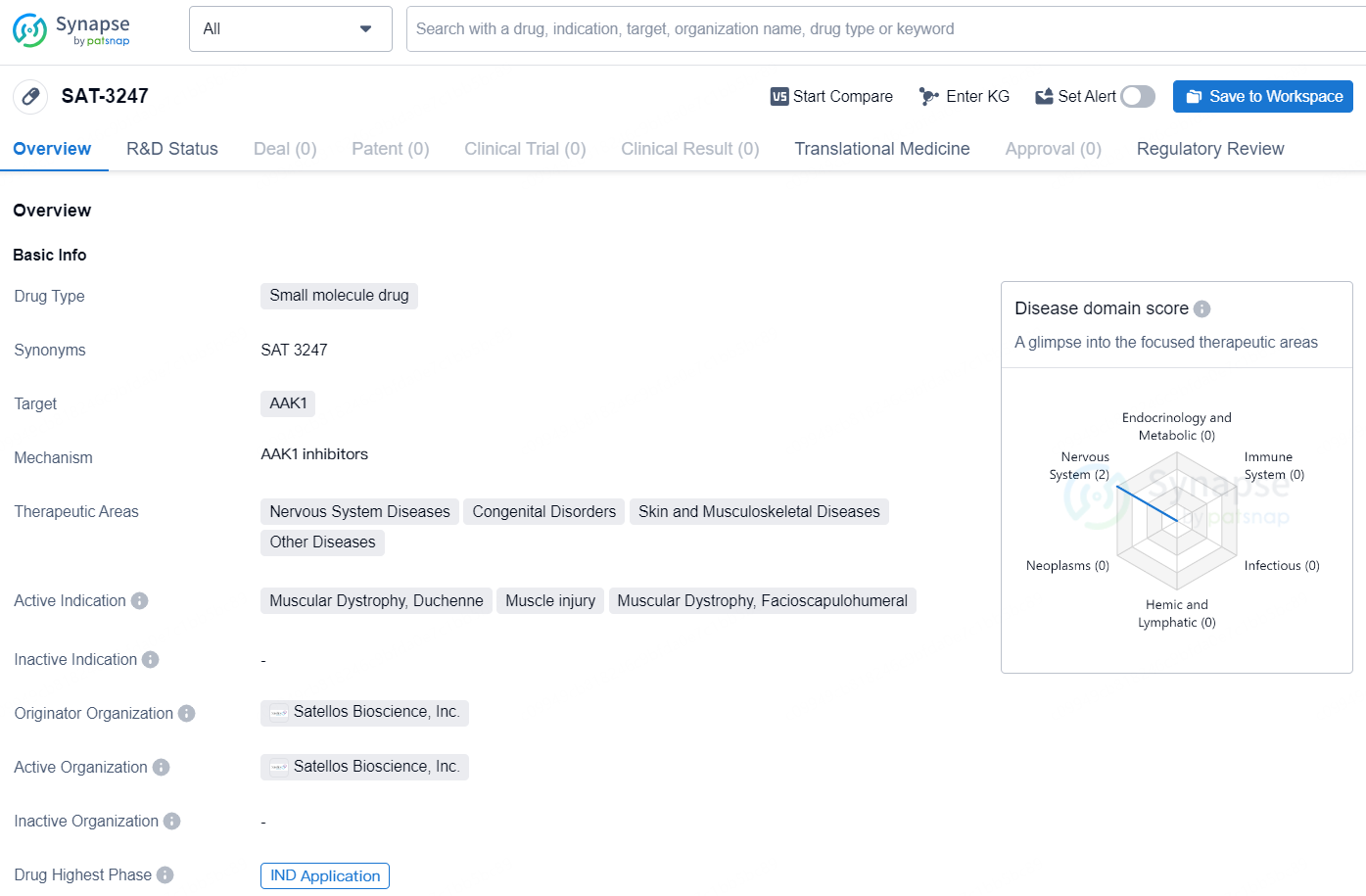
SAT-3247 is a small molecule drug developed by Satellos Bioscience, Inc., targeting the AAK1 protein. SAT-3247 is a proprietary, oral small molecule drug being developed by Satellos as a novel treatment to regenerate skeletal muscle which is lost in Duchenne muscular dystrophy (DMD of Duchenne) and other degenerative or injury conditions. Satellos is advancing SAT-3247 as a potential treatment for DMD, independent of dystrophin and regardless of exon mutation status.