European Union Approval for Mirum Pharmaceuticals’ LIVMARLI to Treat PFIC Patients
Mirum Pharmaceuticals, Inc. revealed that the European Commission has approved the marketing authorization for LIVMARLI® (maralixibat) oral solution, intended for treating progressive familial intrahepatic cholestasis in patients aged three months and above.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The CHMP's positive assessment has led to the approval of LIVMARLI for PFIC, highlighting its significant clinical benefits, including enhanced efficacy and notable improvements in patient care compared to existing PFIC treatments. Furthermore, the COMP's evaluation upheld the Orphan Drug Designation for LIVMARLI in PFIC.
"We are delighted that the European Commission has authorized the marketing of LIVMARLI for PFIC, recognizing the robust data and the valuable treatment opportunity it offers for patients suffering from this rare liver condition," stated Chris Peetz, CEO of Mirum. "Our aim is for LIVMARLI to enhance critical liver parameters and bring better health prospects to young PFIC patients in Europe. We are deeply thankful to the researchers, patients, and families who contributed to this approval."
"The approval of LIVMARLI represents a treatment that has substantial clinical backing, data indicating reduced cholestatic itch, and improved liver health markers, such as decreased serum bile acids—key indicators of better long-term outcomes," said Professor Richard Thompson of King’s College London. "It's reassuring for European physicians to have a new treatment option that can potentially enhance liver health and the quality of life for patients and their families."
Emily Ventura, executive director of the PFIC Network, commented, "The European patient community will significantly benefit from LIVMARLI's approval, which is supported by extensive data showing improvements in the most challenging aspects of the disease. PFIC can drastically alter lives and severely impact patients. The approval provides hope for younger patients to experience relief from cholestasis and its burdens."
LIVMARLI has also received approval from the U.S. Food and Drug Administration for treating cholestatic pruritus in PFIC patients aged five and older. Mirum has submitted a supplemental new drug application to the FDA for a higher concentration formulation used in the MARCH study, aiming to expand the label for younger PFIC patients, with feedback expected within the year.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
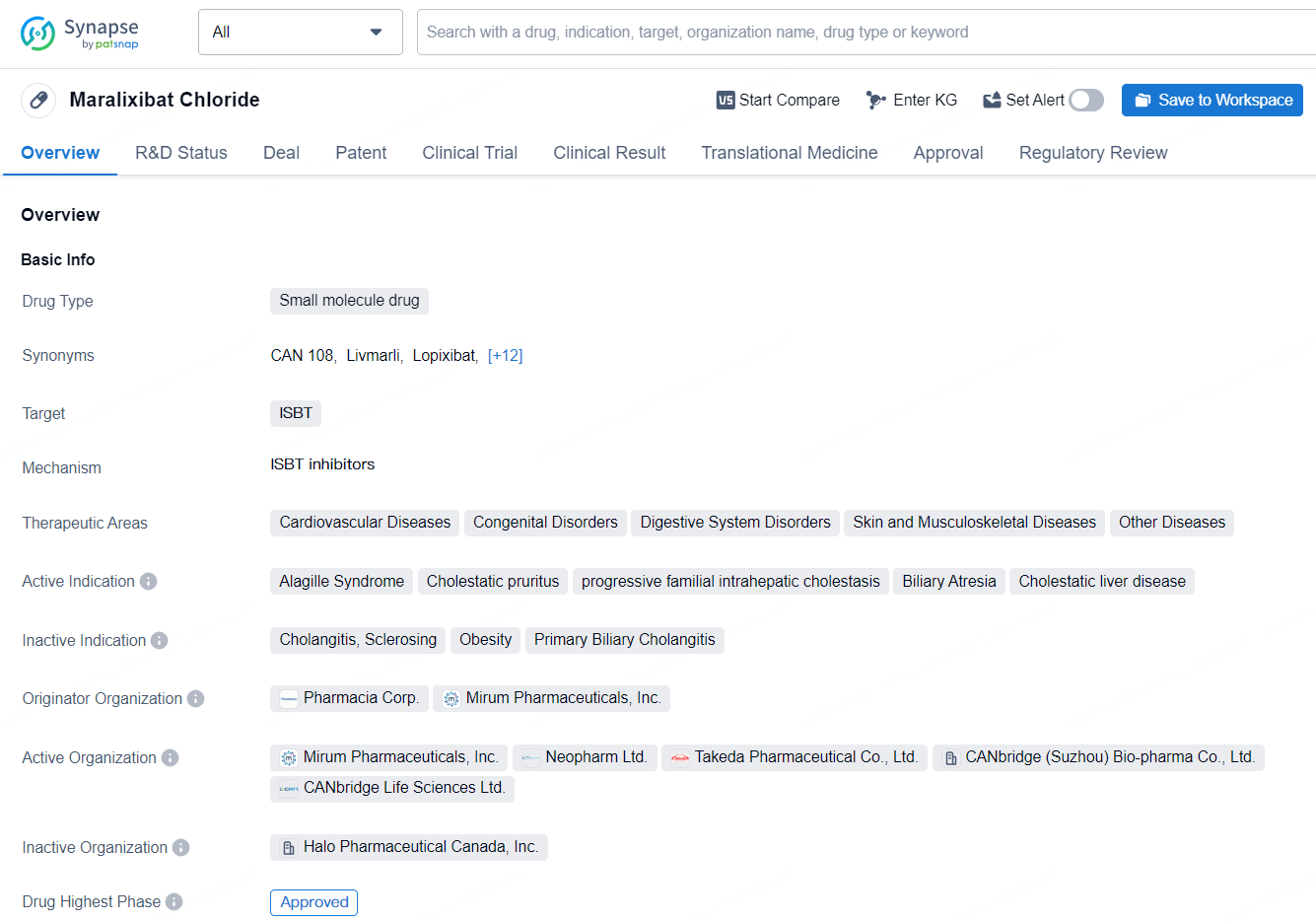
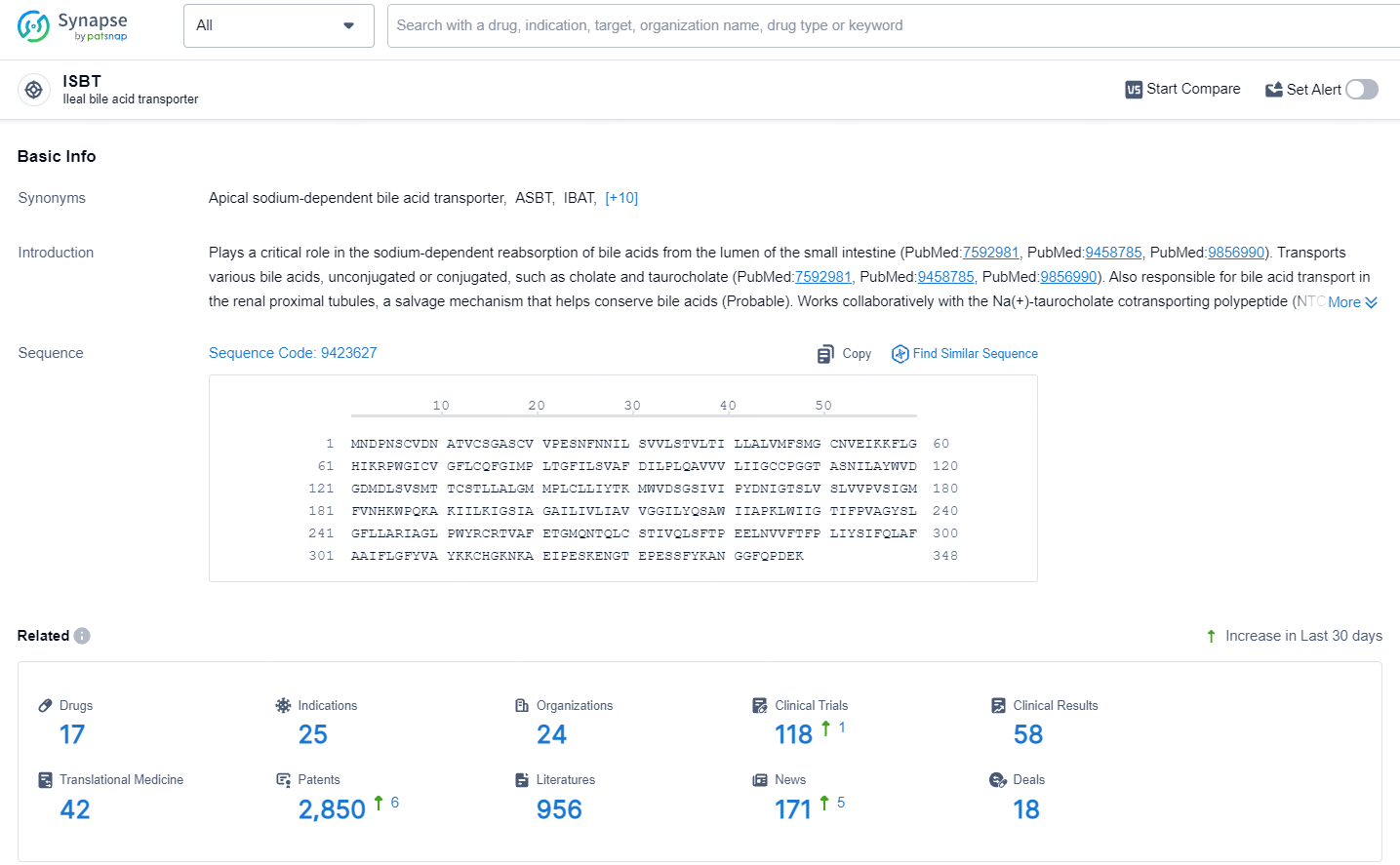
According to the data provided by the Synapse Database, As of July 10, 2024, there are 17 investigational drugs for the ISBT target, including 25 indications, 24 R&D institutions involved, with related clinical trials reaching 118, and as many as 2850 patents.
Maralixibat Chloride is a small molecule drug that targets the ileal bile acid transporter (ISBT). Maralixibat Chloride represents a significant advancement in the treatment of various cholestatic and liver-related conditions. Its approval in both the United States and China, as well as the regulatory designations it has received, underscore the potential impact of this small molecule drug in addressing the needs of patients with these challenging medical conditions.