Expand your analysis with a 360° industry overview!
Patsnap Bio Sequence Database has enhanced the analysis page to include sequence and literature reports now!
Gain instant access to industry insights for your sequence of interest with exportable reports summarizing length and identity distribution, drug and patent activity, IP and literature trends, and more!
The following example uses Semaglutide sequence retrieval to illustrate data transformation from the logical form of search results into the visual form of images. Let's take a closer look at these image effects!
Detailed operation steps:
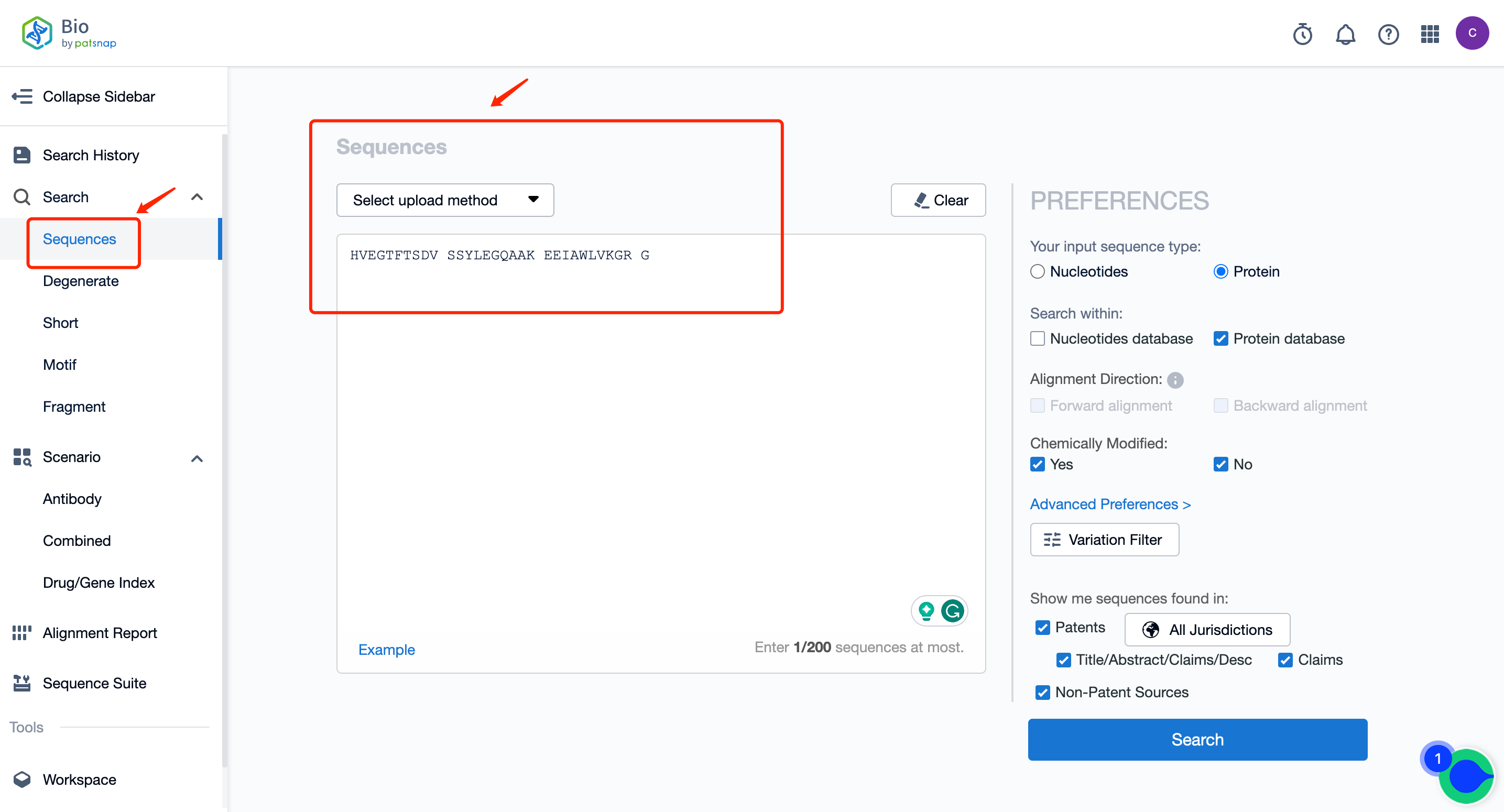
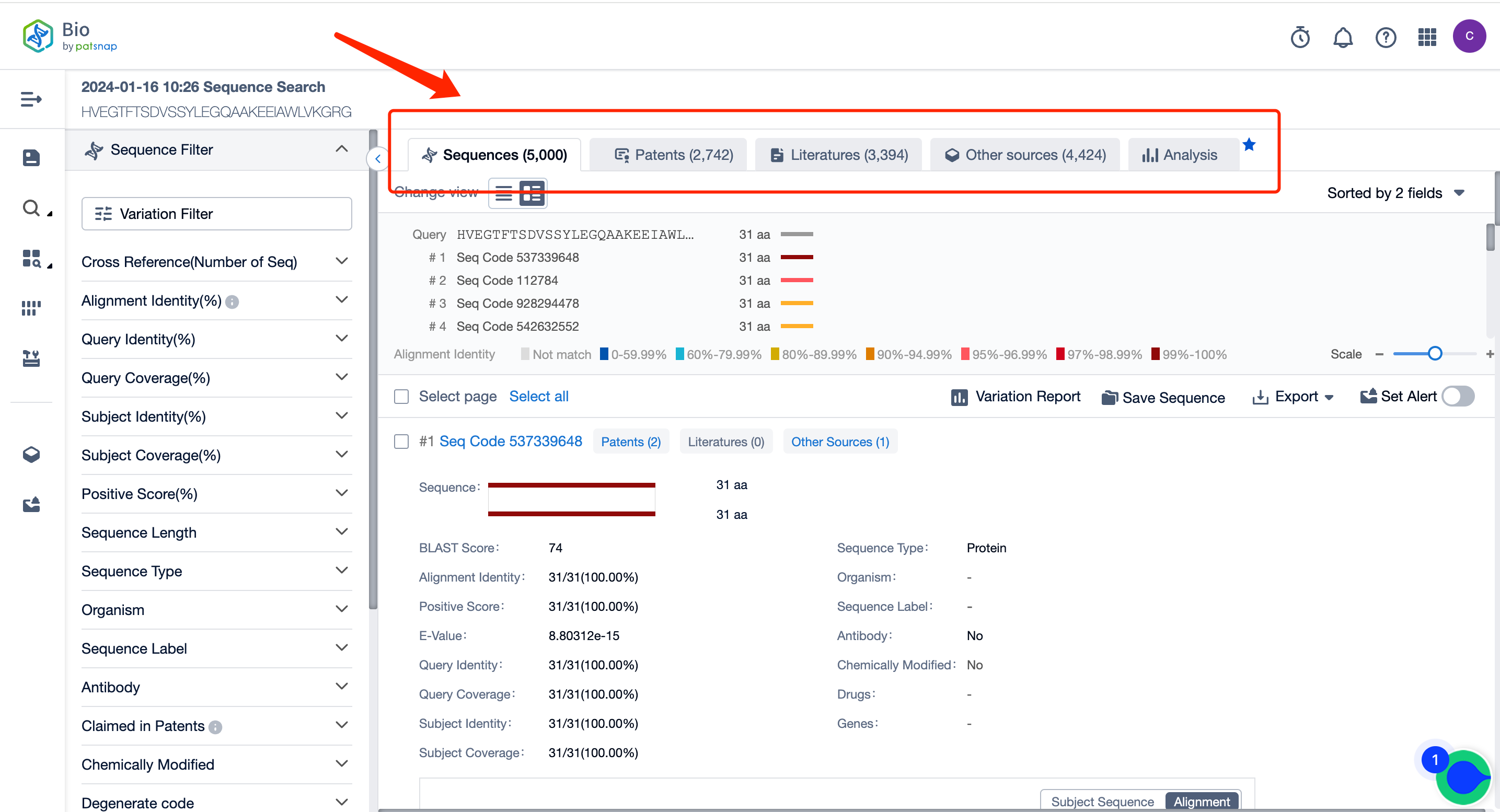
At the homepage of Patsnap Bio Sequence Database, input the sequence of Semaglutide in the regular search: HVEGTFTSDV SSYLEGQAAK EEIAWLVKGR G, click on the search to get its similar sequence data results (including sequence data, patent data, literature data, data from other libraries, and analysis view).
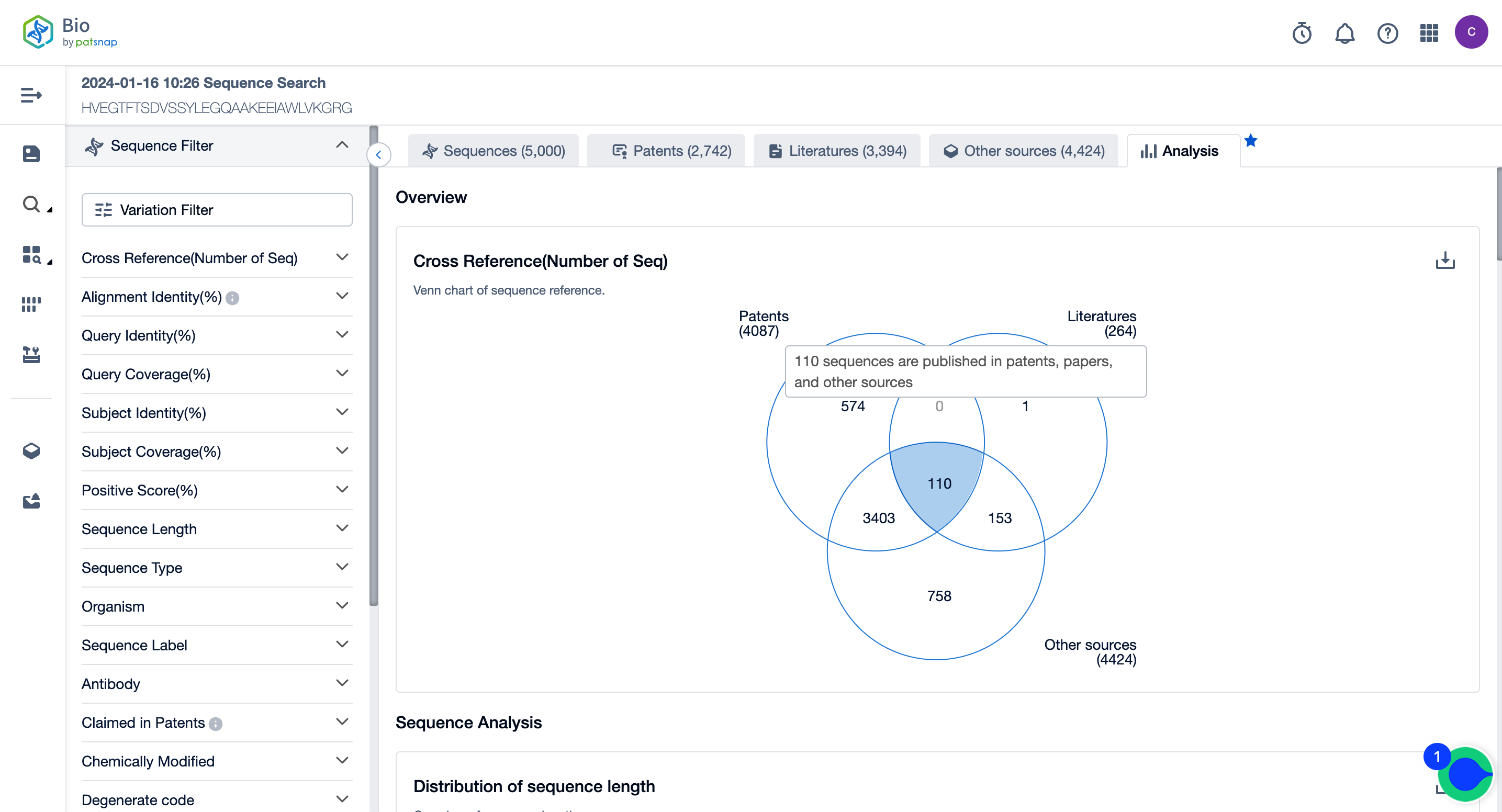
Overview
In the analysis view, the Venn diagram in the overview displays the number of sequences cross-referenced from various sequence sources. 4087 sequences are published in patents, 264 sequences are published in papers, and 4424 sequences are published in other sources, of which 110 sequences are published in patents, papers, and other sources.
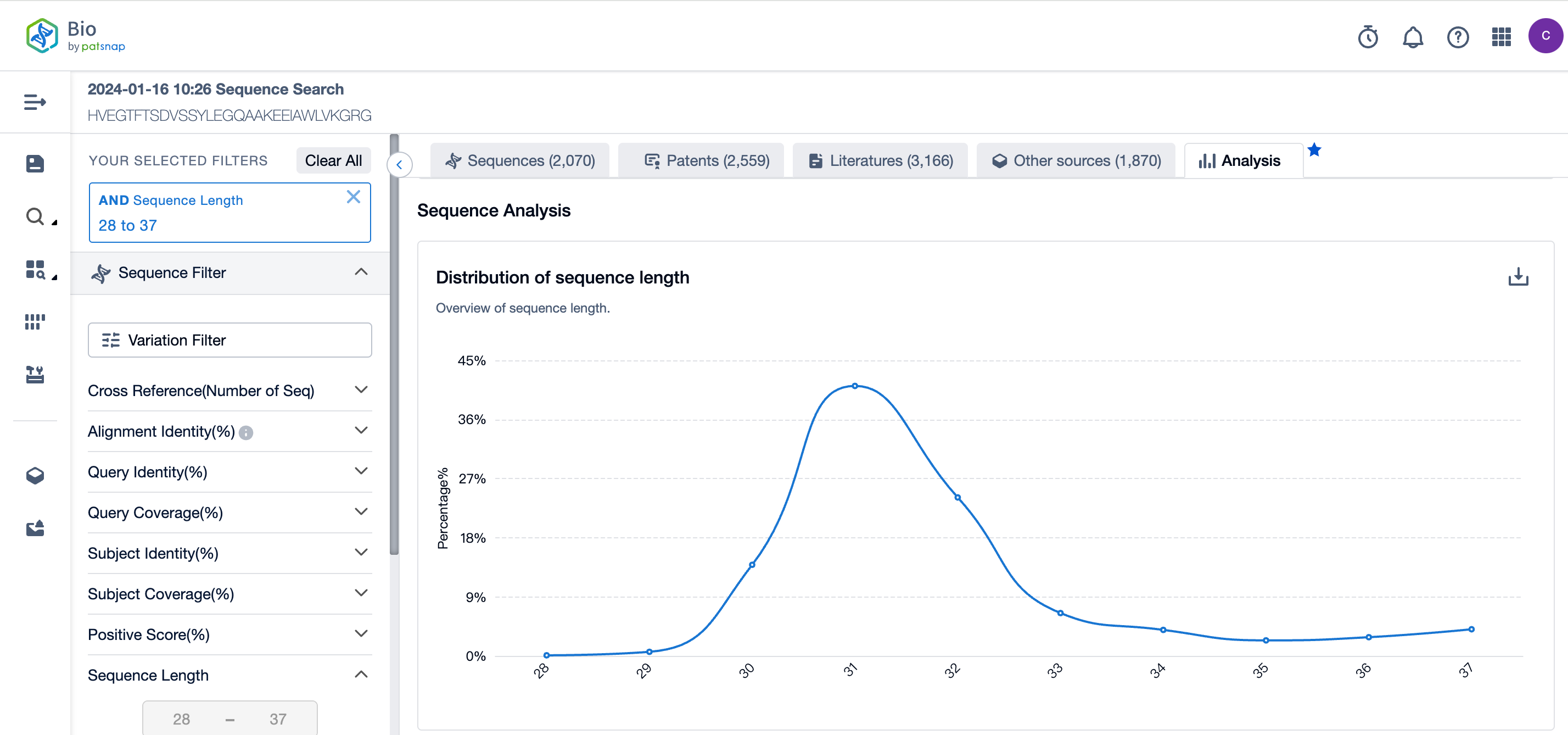
Sequence Analysis
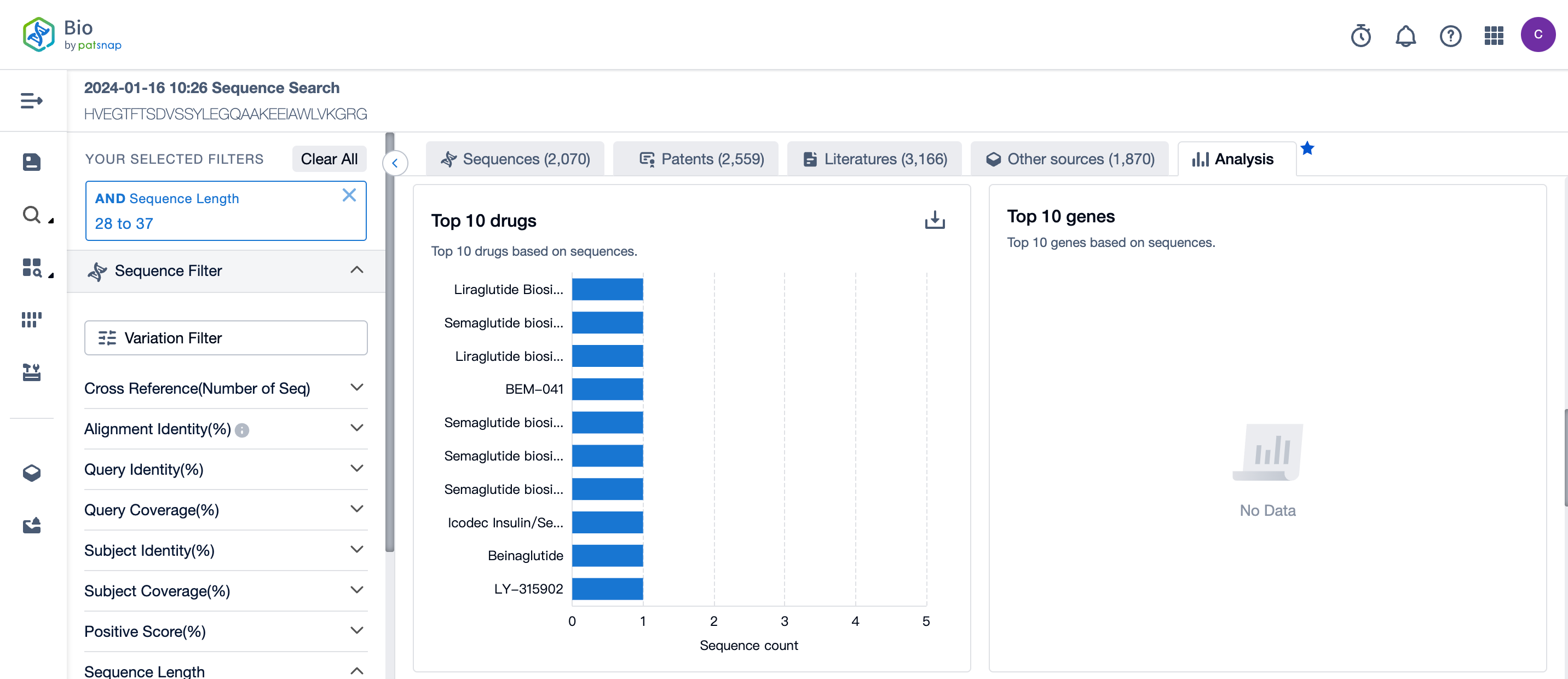
In the analysis view, several aspects of the sequence results are visualized, including the overall distribution of sequence lengths, the overall distribution of alignment identity, the overall distribution of query Identity, the top ten drugs based on sequences, and the top ten genes based on sequences.
From the analysis view, we can easily see the top 10 drugs corresponding to the Semaglutide sequence retrieval results.
Patent Analysis
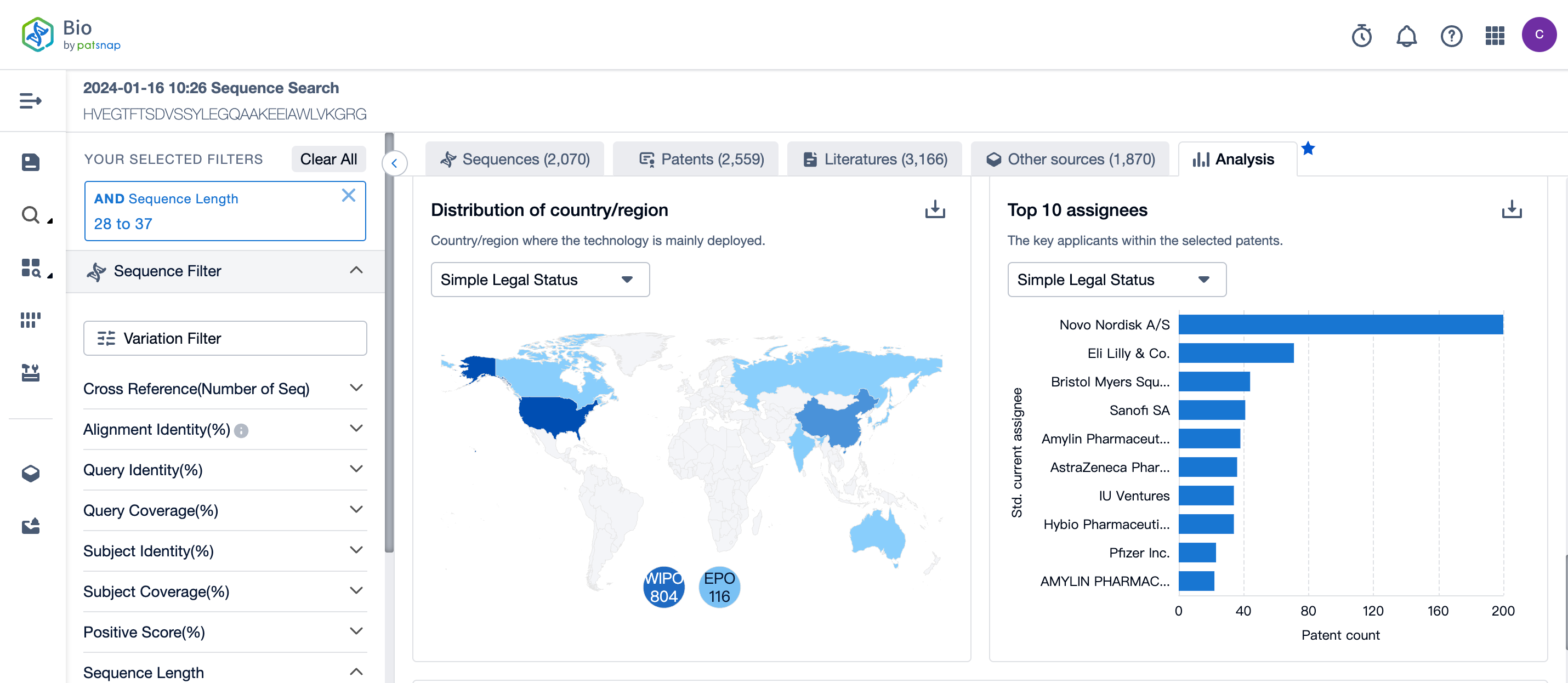
In the analysis view, the patents of the sequence search results are also clearly visualized, including the column chart of the patent application and grant trends in the past ten years, the pie chart of the simple legal status distribution of the selected patents, the main countries/regions of the technology layout, the key of the top 10 applicants among the selected patents, and the top 10 competitive landscape over time.
Literature Analysis
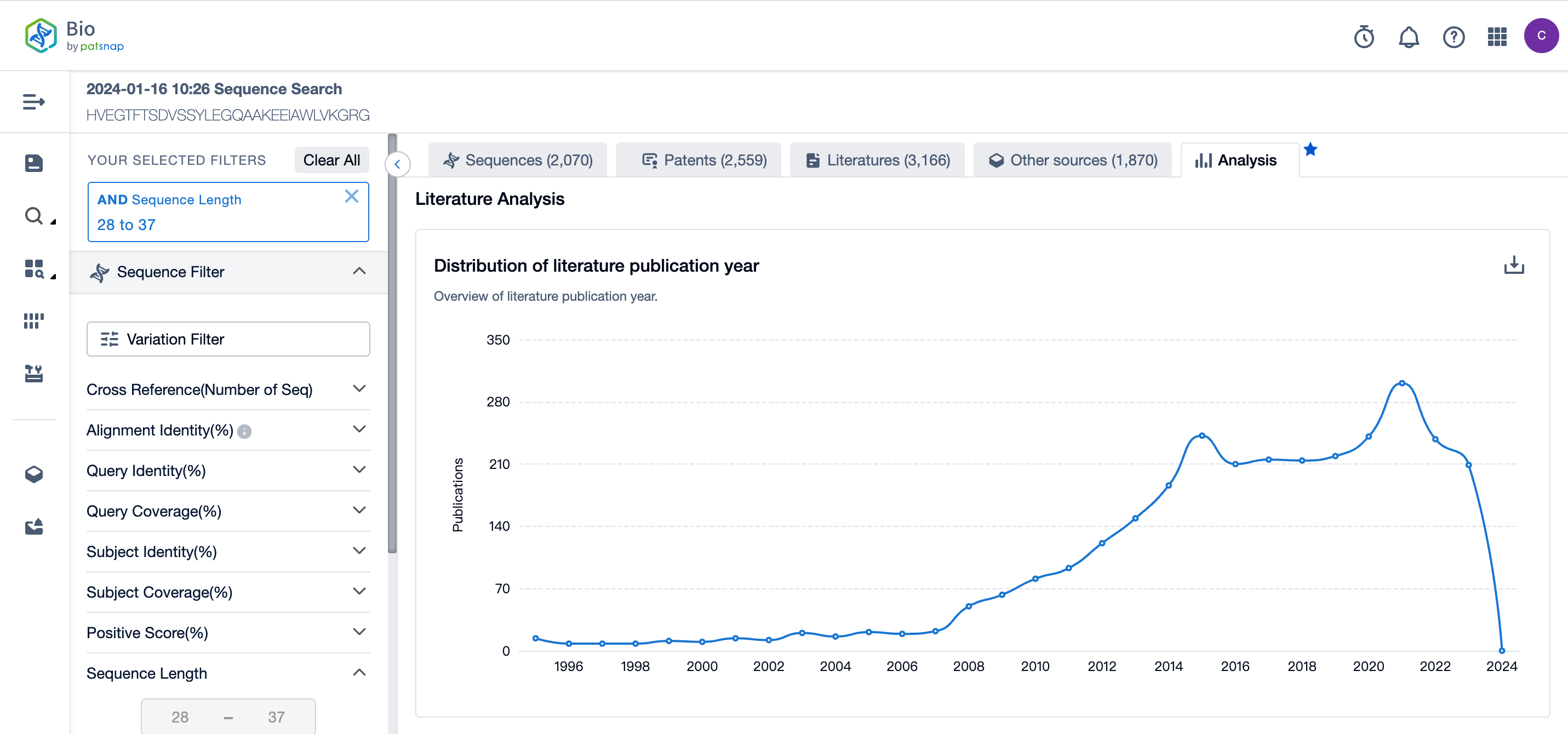
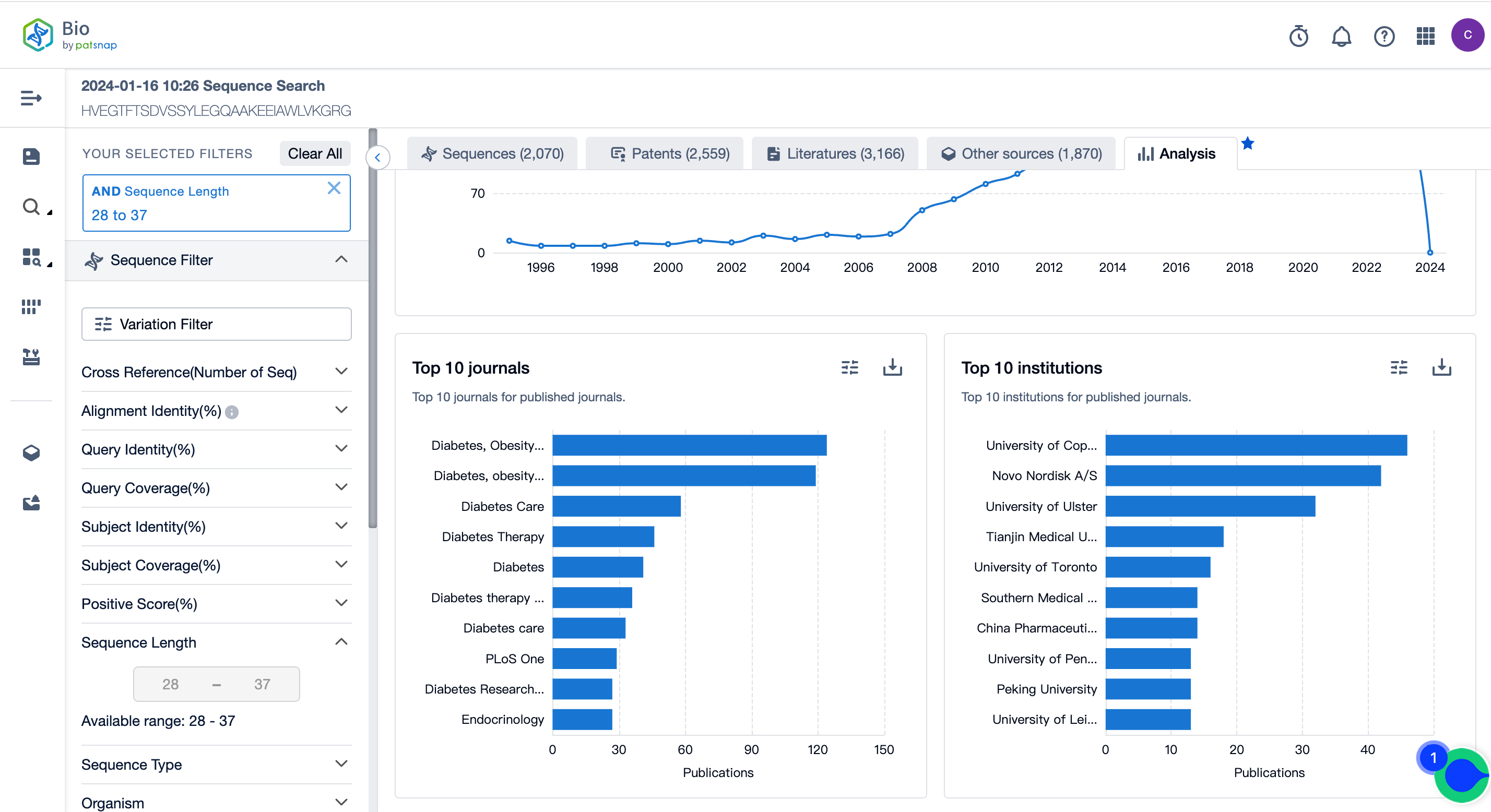
In the analysis view, statistical analyses of the literature results of sequence retrieval are conducted, including the distribution of literature publication years, the top 10 literature publishing journals, and the top 10 literature publishing institutions.
The number of publicly available literature on Semaglutide peptide sequence retrieval rapidly increased from 2008 to 2015 and then remained steady, reaching another peak in 2021. The journal Diabetes, Obesity, and Metabolism ranked first in literature publishing, while the University of Copenhagen ranked first in literature publishing institutions. For more detailed data, refer to the figure below and log in to the Patsnap Bio database.
It is important to note that Patsnap Bio is the most extensive sequence search platform for the Patsnap database. It incorporates AI with human-curated data for comprehensive handling of protein and nucleotide sequence data plucked from global patents, biological periodicals, and public repositories. Essential biological sequences are manually annotated, illuminating structural modifications to provide the most accurate sequence data and boost sequence retrieval efficiency.
Free registration is available for the Bio biological sequence database: https://bio.patsnap.com. Act now to expedite your sequence search tasks.