Exploring cysteamine hydrochloride's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Cysteamine hydrochloride's R&D Progress
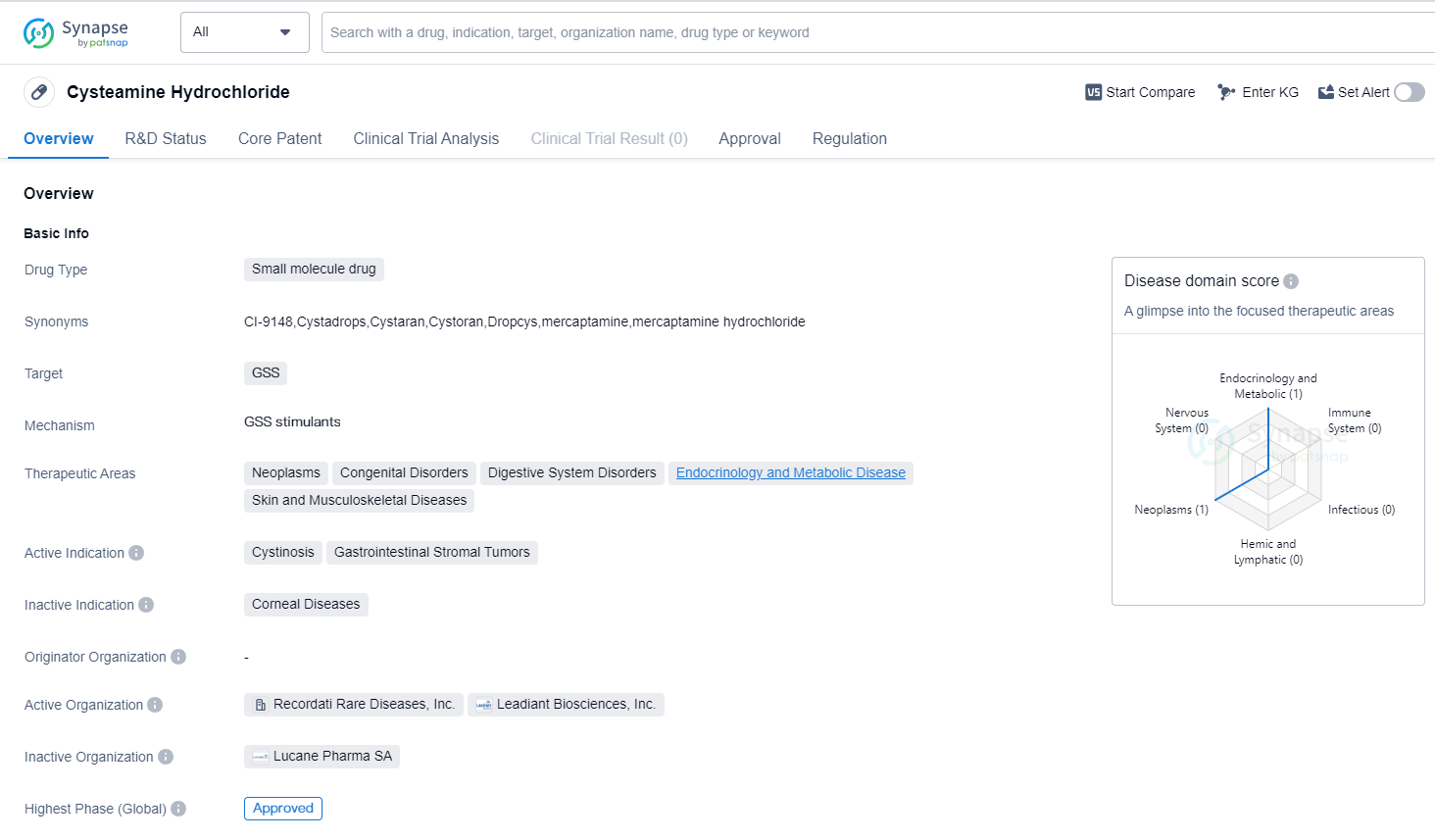
Cysteamine Hydrochloride is a small molecule drug that is primarily used to target the enzyme GSS (gamma-glutamylcysteine synthetase). This drug has been approved for use in the treatment of various therapeutic areas, including neoplasms, congenital disorders, digestive system disorders, endocrinology and metabolic disease, as well as skin and musculoskeletal diseases.
One of the active indications for Cysteamine Hydrochloride is cystinosis, a rare genetic disorder that affects the transport of cystine in the body. This drug has shown efficacy in reducing cystine accumulation in cells and has been approved for the treatment of cystinosis.
Additionally, Cysteamine Hydrochloride has also been approved for the treatment of gastrointestinal stromal tumors (GISTs). GISTs are a type of tumor that originates in the digestive tract, and this drug has demonstrated effectiveness in inhibiting the growth of these tumors.
After completing successful clinical trials and receiving regulatory approval, Cysteamine Hydrochloride has now reached its highest level of development and is approved for use. The drug, which was first approved in the United States in October 2012, has been designated as an orphan drug. This means that it is specifically meant to treat rare diseases or conditions that affect only a small number of patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for cysteamine hydrochloride: GSS stimulants
Glutathione S-transferase stimulants are substances or compounds that enhance the activity or production of glutathione S-transferase (GSS). GSS is an enzyme that plays a crucial role in detoxification processes within the body. It is involved in the conjugation of glutathione, a tripeptide composed of three amino acids, with various toxic substances, drugs, and metabolites, making them more water-soluble and easier to eliminate from the body.
From a biomedical perspective, GSS stimulants can refer to drugs or compounds that increase the expression or activity of GSS enzymes. These stimulants can potentially enhance the body's ability to detoxify harmful substances or drugs, leading to improved drug metabolism and elimination. They may be used in certain medical conditions where enhanced detoxification is desired, such as in cases of drug overdose or exposure to toxic chemicals.
It is important to note that the term "GSS stimulants" is not a widely recognized or established term in biomedicine. Therefore, it is advisable to consult scientific literature or medical professionals for specific information on any potential GSS stimulants and their applications.
Drug Target R&D Trends for cysteamine hydrochloride
GSS, or Glutathione S-transferase, is an essential enzyme found in the human body that plays a crucial role in detoxification processes. It acts as a catalyst in the conjugation of glutathione with various harmful substances, such as toxins, drugs, and carcinogens, making them more water-soluble and easier to eliminate from the body. GSS also helps in the protection of cells against oxidative stress by neutralizing harmful free radicals. This enzyme is involved in maintaining cellular homeostasis and is vital for overall health and well-being. Understanding the role of GSS is important in developing strategies to enhance detoxification pathways and combat oxidative damage.
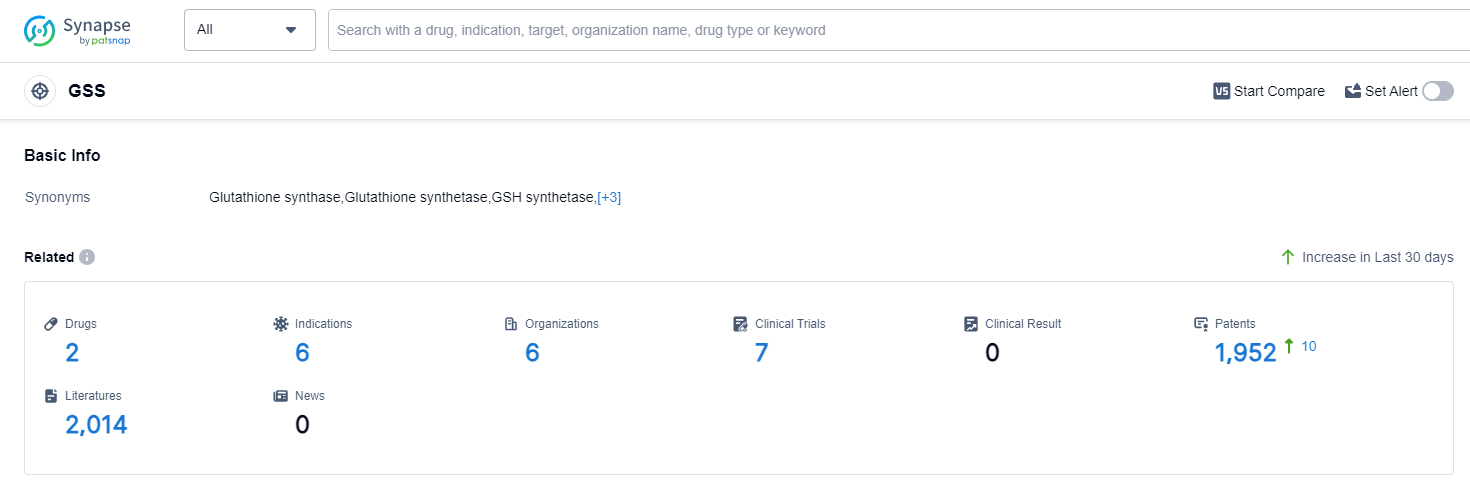
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 2 GSS drugs worldwide, from 6 organizations, covering 6 indications, and conducting 7 clinical trials.
Overall, the target GSS presents opportunities for further research and development, particularly in the areas of small molecule drugs and indications that are still in the inactive phase. Further analysis and monitoring of the competitive landscape and future developments in this target are recommended to stay updated on the latest advancements in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Cysteamine Hydrochloride is a small molecule drug that targets the enzyme GSS. It has been approved for use in the treatment of various therapeutic areas, including cystinosis and gastrointestinal stromal tumors. The drug received its first approval in the United States in 2012 and is regulated as an orphan drug.