Exploring Elacestrant Hydrochloride: A Novel ERα-Targeting Drug for Advanced Cancer Treatment
Elacestrant hydrochloride is a small molecule drug that targets ERα and is used in the treatment of various therapeutic areas including neoplasms, skin and musculoskeletal diseases, nervous system diseases, and digestive system disorders.
The originator organization of Elacestrant hydrochloride is Eisai Co., Ltd. The drug has achieved its highest phase of development globally, with approval obtained in 2023. As of now, the first approval of Elacestrant hydrochloride was granted in the United States. The regulation for this drug includes priority review and fast track status.
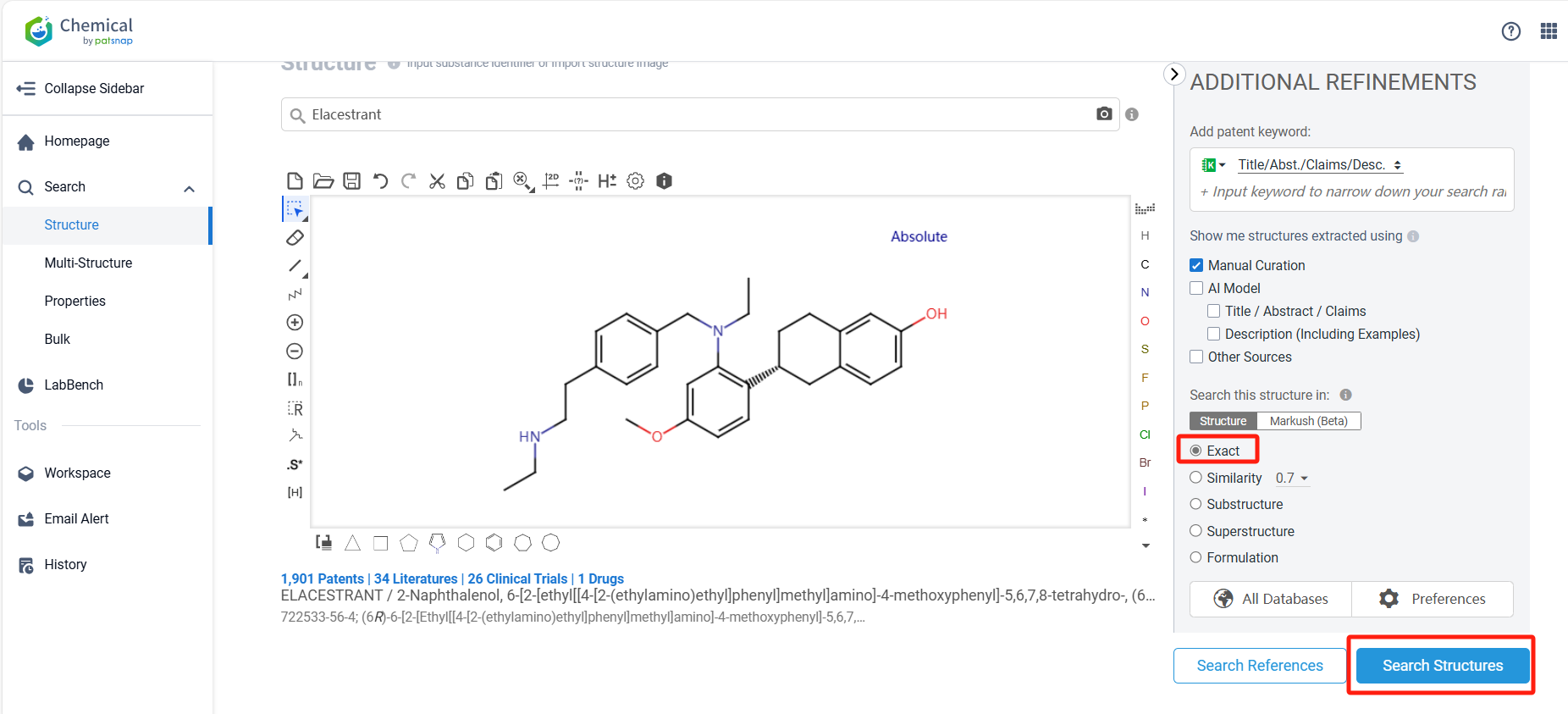
Below, we will use the drug Elacestrant as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
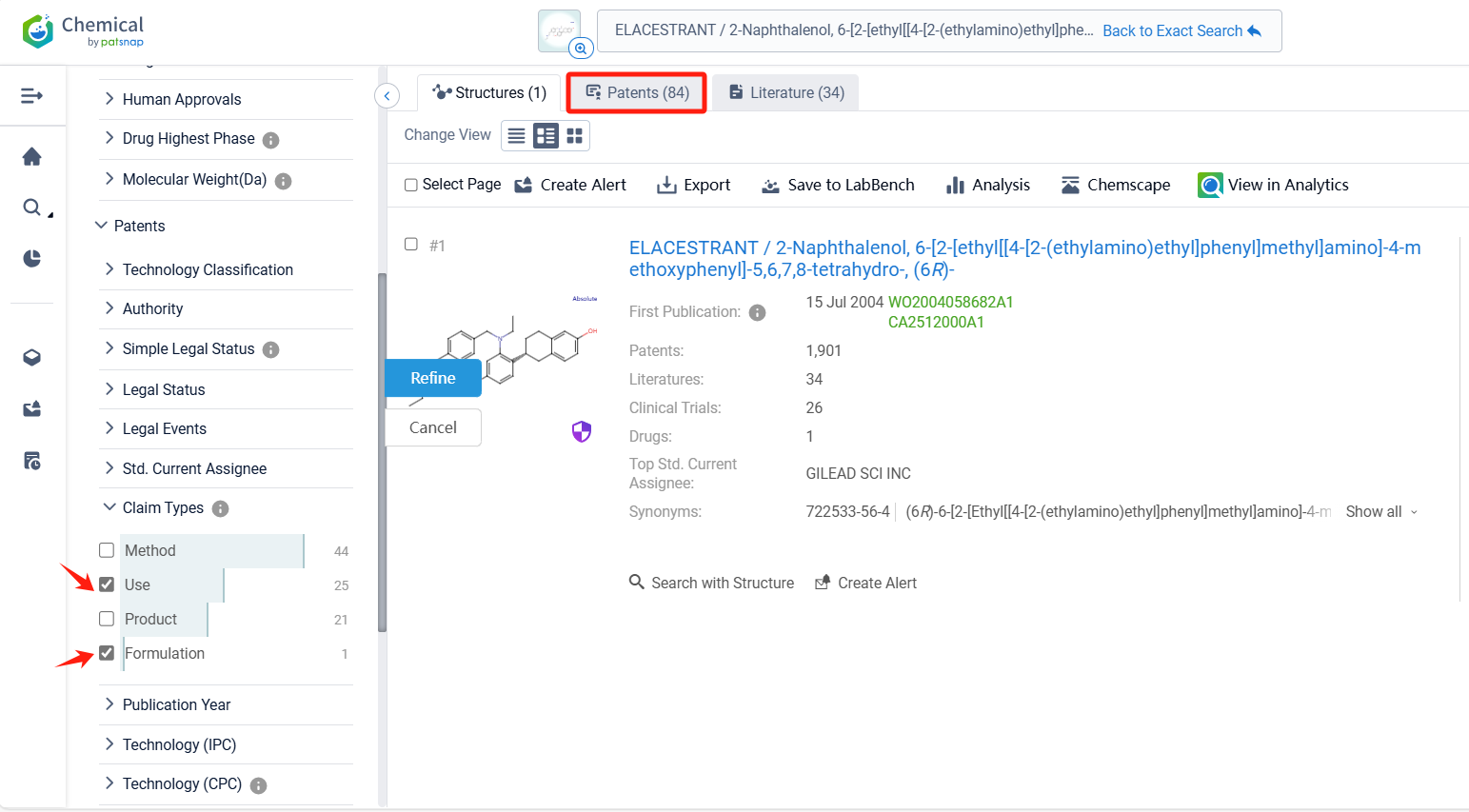
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Elacestrant (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 84 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
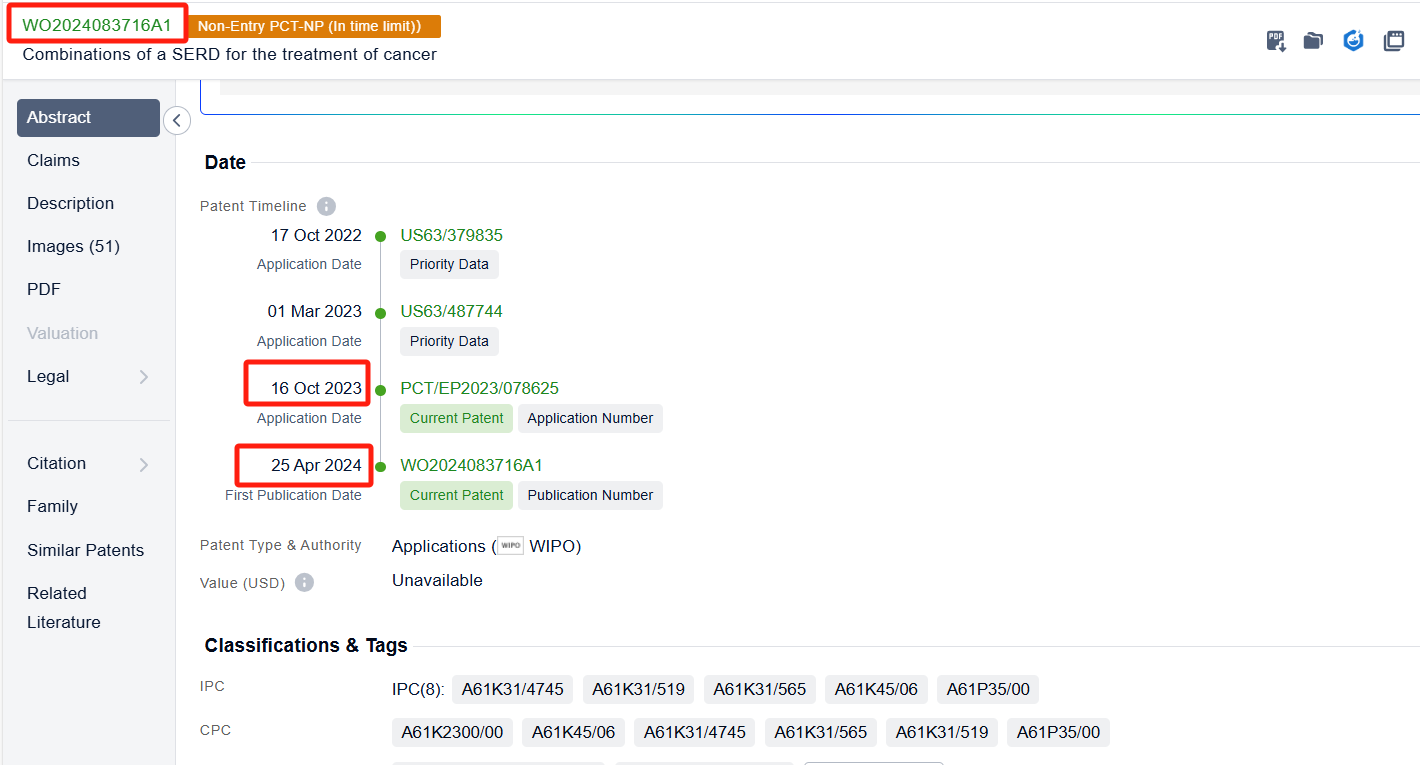
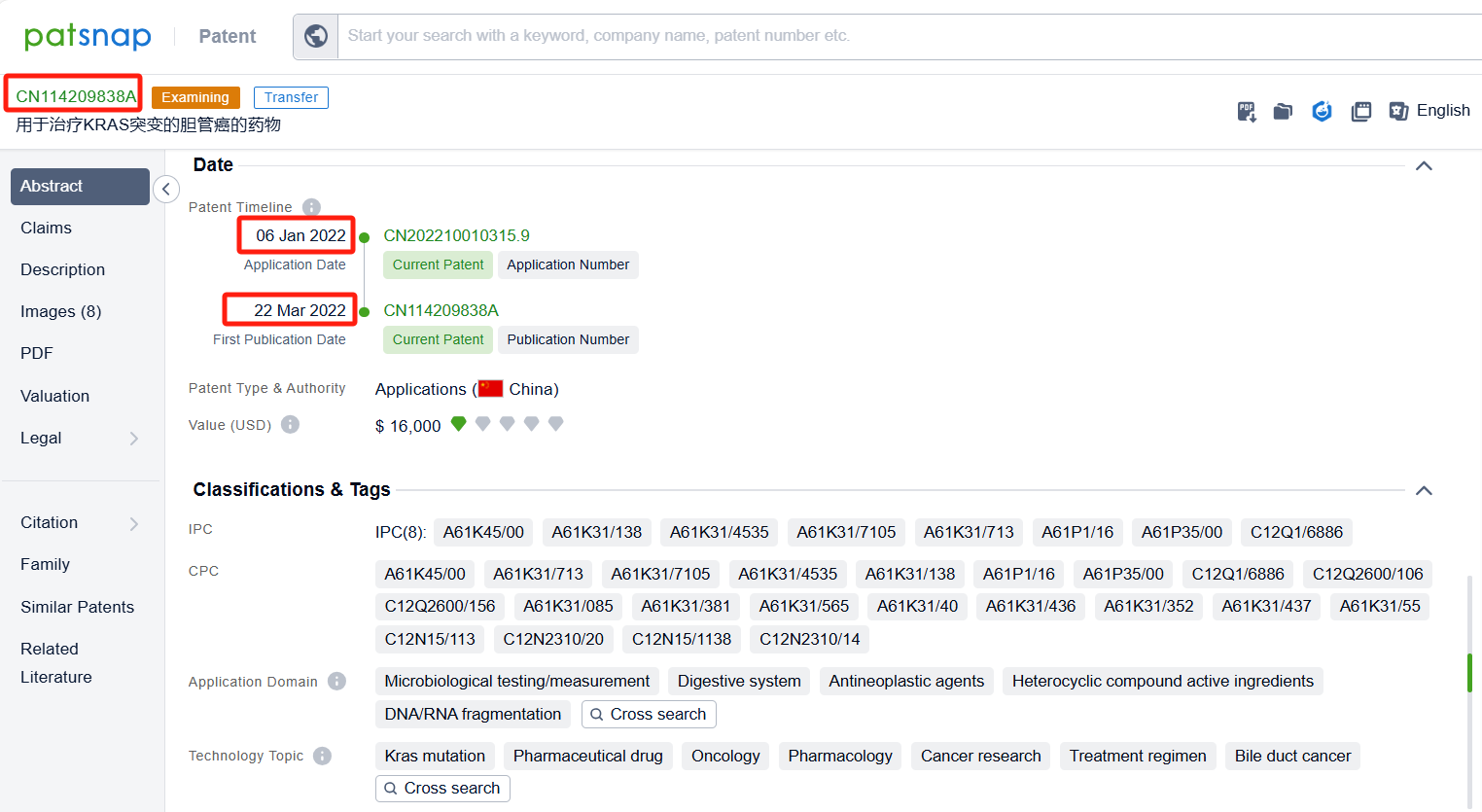
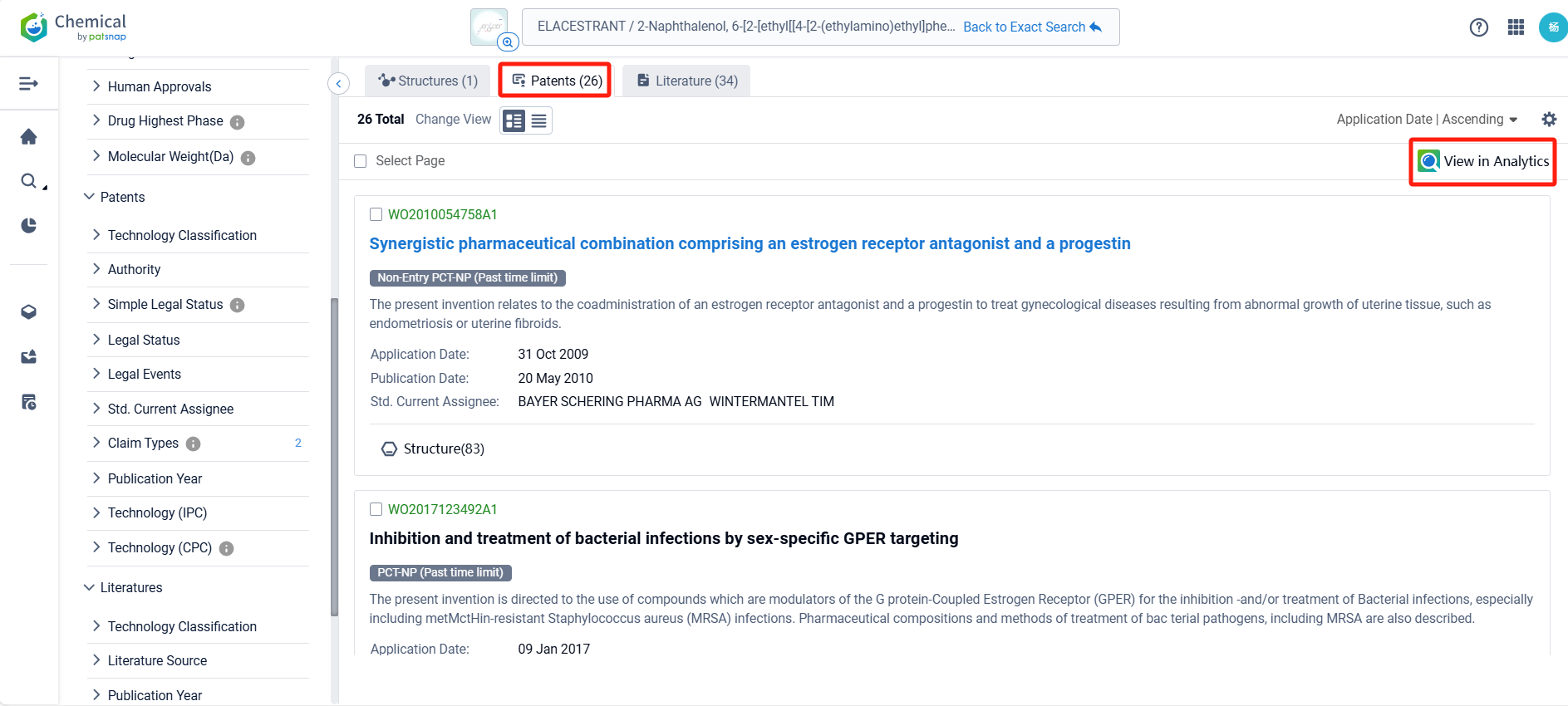 In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Elacestrant. By reviewing the aforementioned patents, we can observe that ASTRAZENECA AB's international patent WO2024083716A1 (application date 20231016, publication date 20240425) describes a method of treating cancer by using a combination of a selective estrogen receptor degrader (SERD) with an inhibitor of either mTOR, AKT, or CDK4/6. The use of these drugs in combination can enhance the anti-proliferative effects of the treatment. Additionally, Genomicare Holdings Co., Ltd.'s patent CN114209838A (application date 20220106, publication date 20220322) relates to a method for treating cholangiocarcinoma with KRAS mutation by an ER inhibitor (Elacestrant). The invention discovered the correlation between KRAS mutations and the ESR1 gene, and confirmed that there is a correlation between KRAS mutations and estrogen pathway activation. Through iCSDB database query, it was found that after knocking out the ESR1 gene, the growth of cell lines with KRAS mutations was more significantly inhibited than KRAS wild-type.
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Elacestrant. By reviewing the aforementioned patents, we can observe that ASTRAZENECA AB's international patent WO2024083716A1 (application date 20231016, publication date 20240425) describes a method of treating cancer by using a combination of a selective estrogen receptor degrader (SERD) with an inhibitor of either mTOR, AKT, or CDK4/6. The use of these drugs in combination can enhance the anti-proliferative effects of the treatment. Additionally, Genomicare Holdings Co., Ltd.'s patent CN114209838A (application date 20220106, publication date 20220322) relates to a method for treating cholangiocarcinoma with KRAS mutation by an ER inhibitor (Elacestrant). The invention discovered the correlation between KRAS mutations and the ESR1 gene, and confirmed that there is a correlation between KRAS mutations and estrogen pathway activation. Through iCSDB database query, it was found that after knocking out the ESR1 gene, the growth of cell lines with KRAS mutations was more significantly inhibited than KRAS wild-type.
Overall, Elacestrant hydrochloride is a novel small molecule drug developed by Eisai Co., Ltd. targeting ERα, and has shown promising results in the treatment of various indications related to breast cancer and other related diseases. With its approval in the United States and ongoing phase 1 development in China, Elacestrant hydrochloride represents a significant advancement in the field of biomedicine and pharmaceuticals, offering potential benefits to patients with advanced breast cancer and related conditions. The drug has received special regulatory considerations, including priority review and fast track status, reflecting its potential to address unmet medical needs and expedite its availability to patients in need.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.