SystImmune Gets FDA Approval for BL-M17D1 IND Application for Advanced Solid Tumors
SystImmune, Inc (SystImmune), a biotechnology firm in the clinical development phase, has revealed that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) submission for BL-M17D1, which is an antibody-drug conjugate (ADC) utilizing innovative linker and payload technology. This IND approval allows the company to commence a Phase 1 clinical trial, designated BL-M17D1-ST-101, aimed at assessing the safety, tolerability, pharmacokinetics, and initial efficacy of BL-M17D1 in patients suffering from advanced or metastatic solid tumors within the United States.
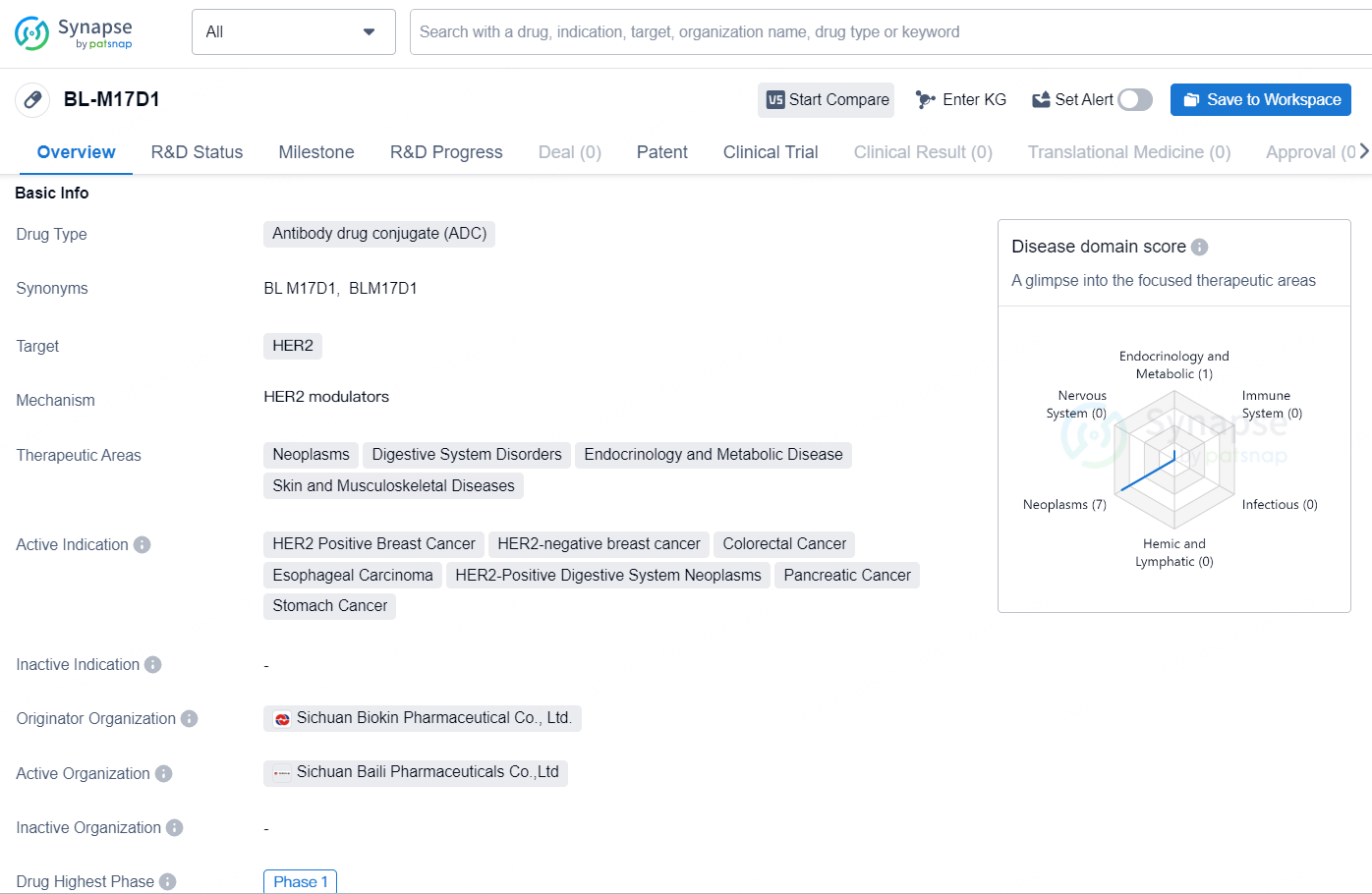
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Aside from HM17321, Hanmi has launched a separate investigation that examines the combined effects of HM17321 when paired with HM15275, a novel anti-obesity drug (LA-GLP/GIP/GCG), and semaglutide. The findings from this combination regimen showed a notable reduction in body weight and fat mass in comparison to results from singular treatments, effectively addressing the muscle degradation often linked with significant weight loss therapies.
These findings underscore HM17321's role in enhancing the weight loss experience by facilitating fat loss, increasing muscle mass, and improving muscle performance, thus providing advantages in both independent and combination treatment regimens.
Hanmi also disclosed further nonclinical data regarding HM15275 (LA-GLP-1/GIP/GCG), which was first discussed at the American Diabetes Association (ADA) conference. This promising anti-obesity candidate is said to potentially achieve more than 25% weight loss while minimizing muscle loss, in addition to offering benefits for metabolic health. At the 2024 ObesityWeek, Hanmi highlighted the mechanism of HM15275, which fosters positive metabolic alterations and enhances energy metabolism when used alongside dietary management. These characteristics set HM15275 apart from current incretin-based obesity treatments, which primarily focus on appetite suppression.
Currently, HM15275 is in Phase 1 clinical trials in the United States, with plans to progress to Phase 2 trials by 2025, positioning it as a prominent candidate in the field of obesity management.
Reflecting on these significant research and development achievements, Dr. Choi stated, “This year, Hanmi has reinforced its leadership in the realm of obesity management through the introduction of HM17321 and HM15275. These advancements continue the momentum initiated by efpeglenatide in our H.O.P project. Our commitment to pioneering research and development will drive substantial progress in addressing obesity, overcoming challenges once viewed as insurmountable.”
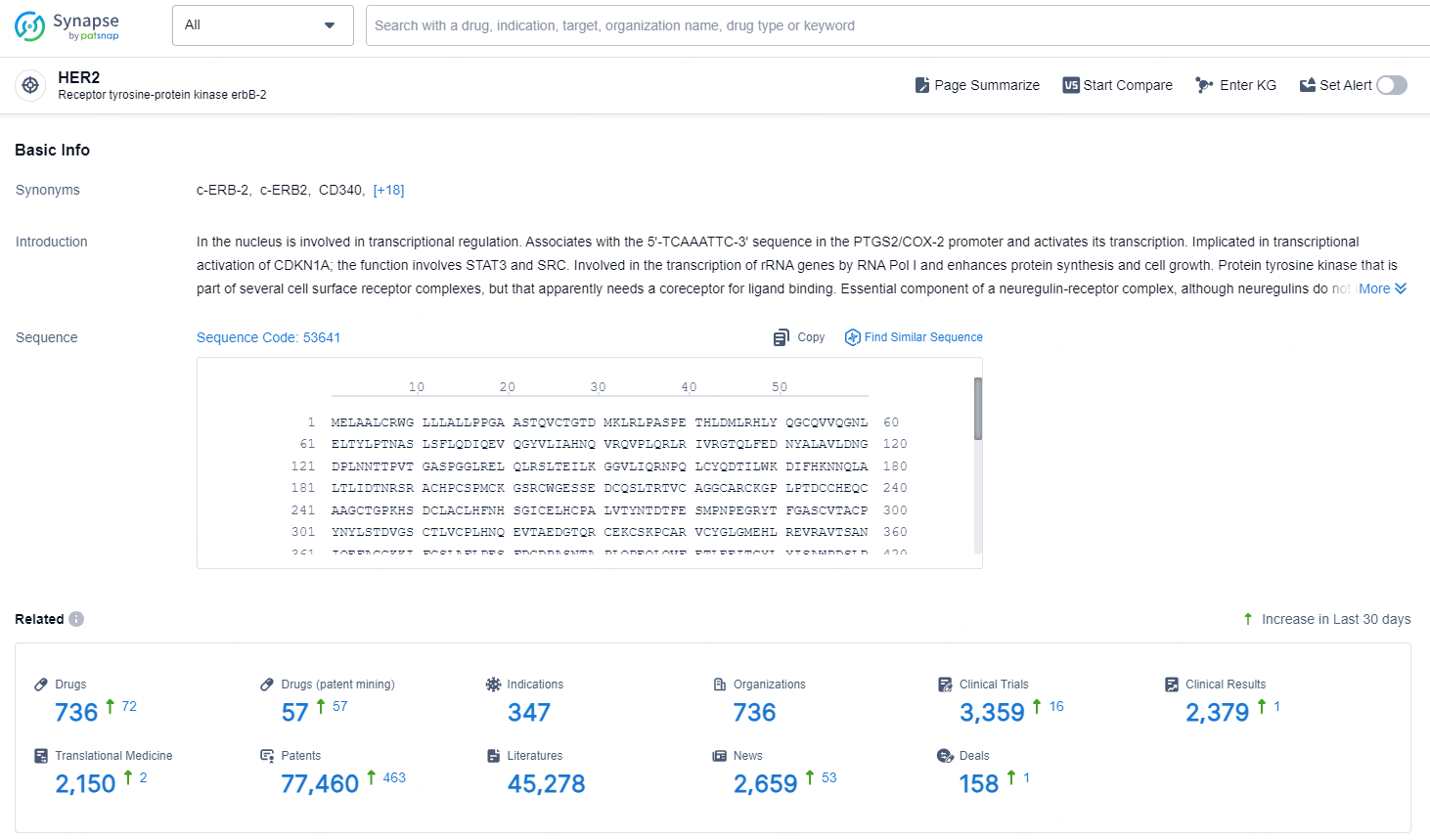
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 11, 2024, there are 736 investigational drugs for the HER2 target, including 347 indications, 736 R&D institutions involved, with related clinical trials reaching 3359, and as many as 77460 patents.
The drug BL-M17D1 is an antibody drug conjugate (ADC) that targets the human epidermal growth factor receptor 2 (HER2). It is being developed for the treatment of various neoplastic and digestive system disorders, as well as endocrinology and metabolic diseases, and skin and musculoskeletal diseases. The active indications for this drug include HER2-positive breast cancer, HER2-negative breast cancer, colorectal cancer, esophageal carcinoma, HER2-positive digestive system neoplasms, pancreatic cancer, and stomach cancer.