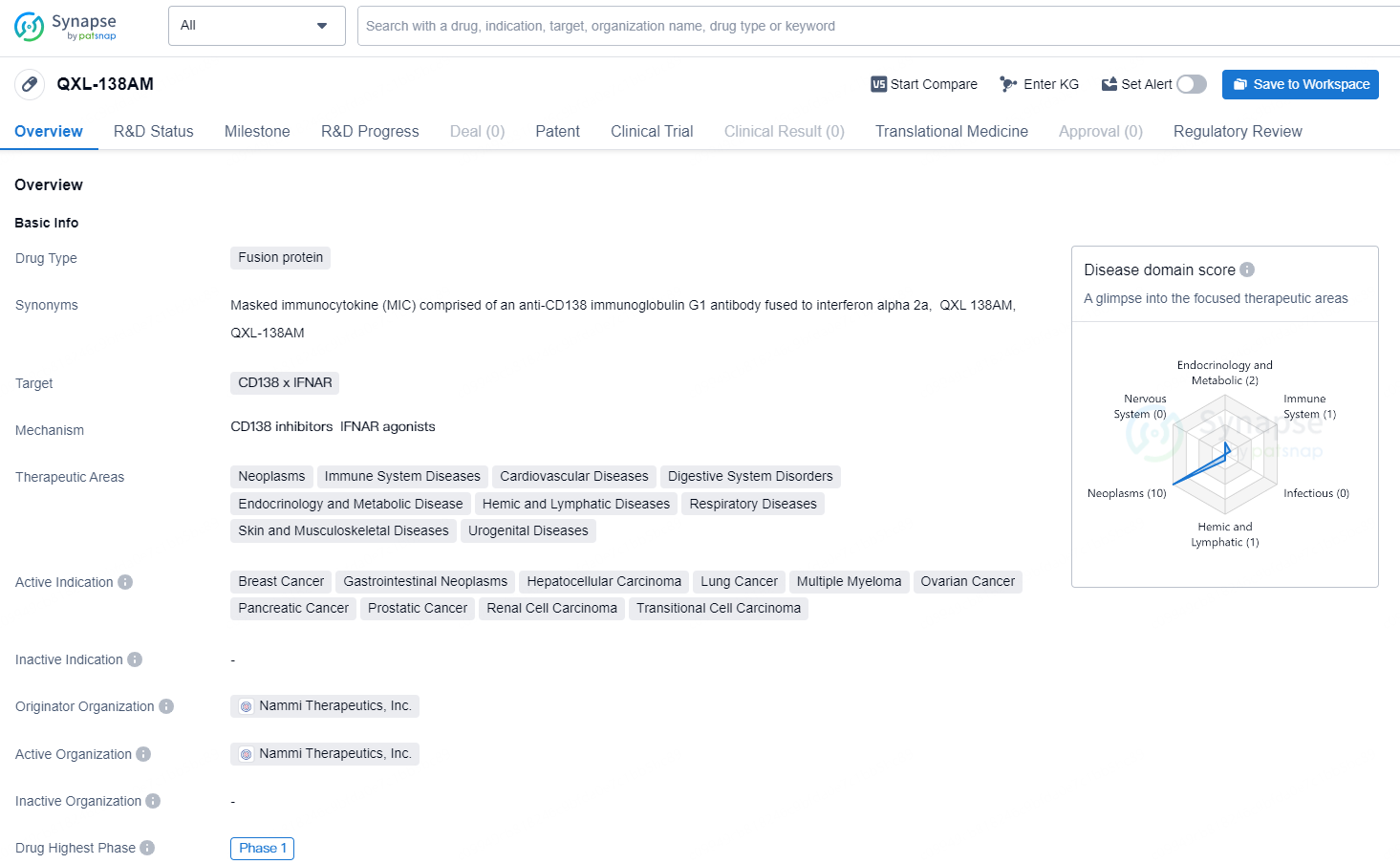
First Patient Administrated with QXL138AM in Phase 1 Trial by Nammi Therapeutics
Nammi Therapeutics, Inc. (Nammi), a research-focused company working in the field of immuno-oncology, has a varied pipeline developed through its Masked-Immunocytokine (MIC) and Nammisome platforms. The company has announced that the first participant has been dosed in its initial human Phase 1 trial (NCT06582017).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The Phase 1 trial is structured as a two-part, open-label, multicenter investigation aimed at enrolling around 100 participants diagnosed with advanced CD138-expressing tumors. The first part will focus on assessing the safety and tolerability of increasing doses of QXL138AM, alongside secondary objectives that will measure pharmacokinetics and immunogenicity. The second part will consist of dose expansion involving three groups (two solid tumor indications with a high prevalence of CD138 and multiple myeloma), with primary outcomes targeting safety and tolerability, and secondary outcomes evaluating antitumor efficacy. The trial will be executed at research sites throughout the United States.
Although the specific solid tumor indications for expansion remain unspecified, QXL138AM has received Orphan Drug Designation for Pancreatic Cancer. Additional considerations, such as the prevalence of CD138 expression, preclinical effectiveness, historical data on clinical efficacy from approved Interferon alpha treatments, the level of unmet medical need, and Nammi's clinical insights from Part A, will inform the selection of solid tumor indications for expansion.
“Interferon alpha 2 represents a strong anticancer agent; however, its clinical advantage is hindered by notable toxicity when given systemically. QXL138AM employs Nammi’s masked immunocytokine technology, which allows interferon alpha 2 to be concealed and linked to a tumor-targeting antibody. This antibody localizes QXL138AM to the tumor cell surface, where proteolytic enzymes can unmask and activate Interferon alpha 2, thereby enhancing the therapeutic scope," explained Dr. Dennis Kim, M.D., the chief medical officer overseeing the study.
“We are thrilled to have administered the first dose of QXL138AM at START,” remarked Dr. Drew W. Rasco, Associate Director at The START Center for Cancer Research in San Antonio, TX. “We perceive considerable promise in Nammi’s immunocytokine technology for addressing various cancer types, and we eagerly anticipate collaborating with the Nammi team to advance this innovative therapy in the years ahead.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
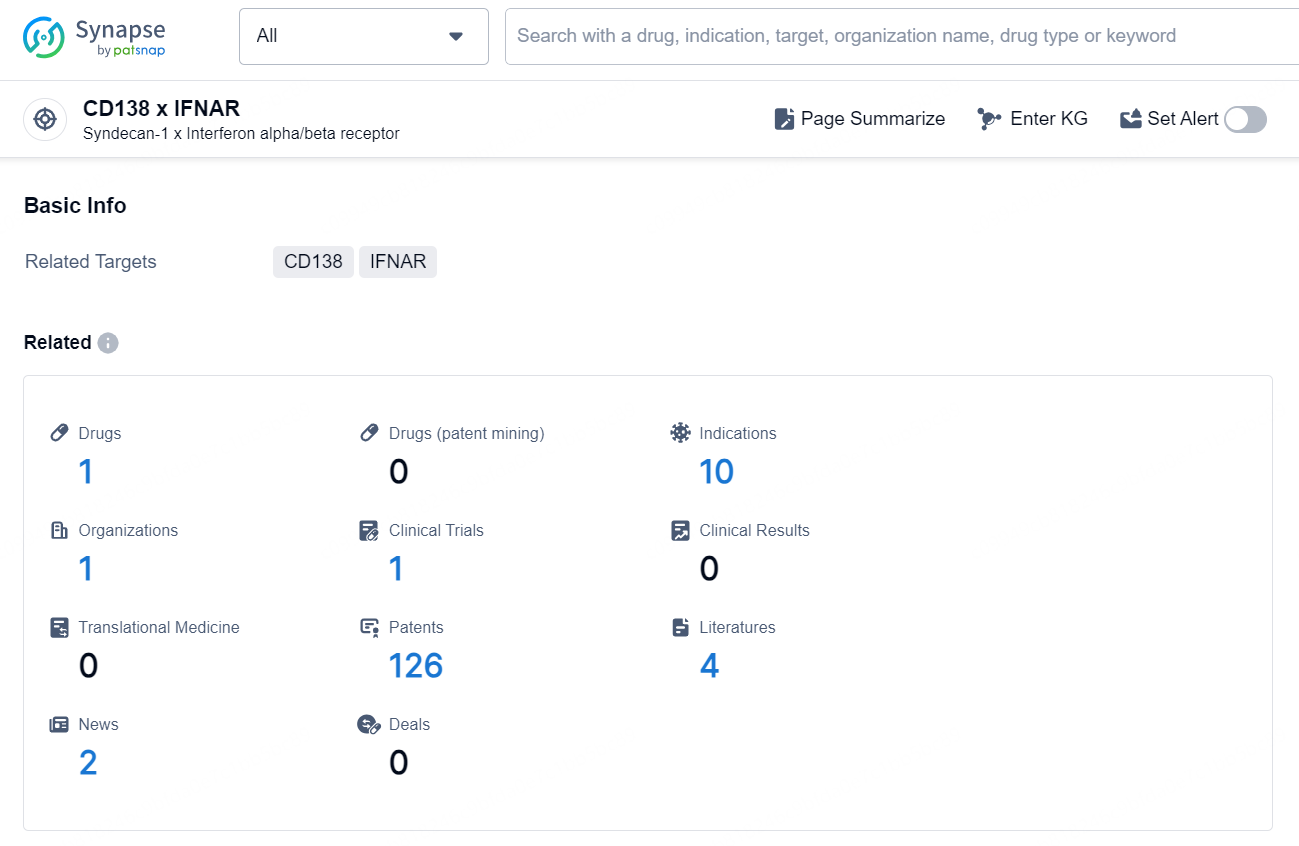
According to the data provided by the Synapse Chemical, As of November 11, 2024, there are 1 investigational drug for the CD138 x IFNAR target, including 10 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 126 patents.
QXL-138AM is a fusion protein drug developed by Nammi Therapeutics, Inc. It targets the CD138 x IFNAR and is currently in the highest phase (Phase 1) of clinical trials globally. The drug falls under the category of orphan drugs and has a wide range of therapeutic areas.